NALP-3 inflammasome silencing attenuates ceramide-induced transepithelial permeability
- PMID: 22169929
- PMCID: PMC3323724
- DOI: 10.1002/jcp.24026
NALP-3 inflammasome silencing attenuates ceramide-induced transepithelial permeability
Abstract
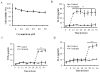
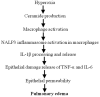
The hallmark of acute lung injury (ALI) is the influx of proinflammatory cytokines into lung tissue and alveolar permeability that ultimately leads to pulmonary edema. However, the mechanisms involved in inflammatory cytokine production and alveolar permeability are unclear. Recent studies suggest that excessive production of ceramide has clinical relevance as a mediator of pulmonary edema and ALI. Our earlier studies indicate that the activation of inflammasome promotes the processing and secretion of proinflammatory cytokines and causes alveolar permeability in ALI. However, the role of ceramide in inflammasome activation and the underlying mechanism in relation to alveolar permeability is not known. We hypothesized that ceramide activates the inflammasome and causes inflammatory cytokine production and alveolar epithelial permeability. To test this hypothesis, we analyzed the lung ceramide levels during hyperoxic ALI in mice. The effect of ceramide on activation of inflammasome and production of inflammatory cytokine was assessed in primary mouse alveolar macrophages and THP-1 cells. Alveolar transepithelial permeability was determined in alveolar epithelial type-II cells (AT-II) and THP-1 co-cultures. Our results reveal that ceramide causes inflammasome activation, induction of caspase-1, IL-1β cleavage, and release of proinflammatory cytokines. In addition, ceramide further induces alveolar epithelial permeability. Short-hairpin RNA silencing of inflammasome components abrogated ceramide-induced secretion of proinflammatory cytokines in vitro. Inflammasome silencing abolishes ceramide-induced alveolar epithelial permeability in AT-II. Collectively, our results demonstrate for the first time that ceramide-induced secretion of proinflammatory cytokines and alveolar epithelial permeability occurs though inflammasome activation.
Copyright © 2011 Wiley Periodicals, Inc.
Figures



Similar articles
-
Mechanisms of pulmonary microvascular endothelial cells barrier dysfunction induced by LPS: The roles of ceramides and the Txnip/NLRP3 inflammasome.Microvasc Res. 2023 May;147:104491. doi: 10.1016/j.mvr.2023.104491. Epub 2023 Jan 26. Microvasc Res. 2023. PMID: 36709858
-
The inflammasome mediates hyperoxia-induced alveolar cell permeability.J Immunol. 2010 May 15;184(10):5819-26. doi: 10.4049/jimmunol.0902766. Epub 2010 Apr 7. J Immunol. 2010. PMID: 20375306 Free PMC article.
-
Gas6 induces AIM to suppress acute lung injury in mice by inhibiting NLRP3 inflammasome activation and inducing autophagy.Front Immunol. 2025 Feb 17;16:1523166. doi: 10.3389/fimmu.2025.1523166. eCollection 2025. Front Immunol. 2025. PMID: 40034700 Free PMC article.
-
MANF inhibits NLRP3 inflammasome activation by competitively binding to DDX3X in paraquat-stimulated alveolar macrophages.Ecotoxicol Environ Saf. 2024 Nov 15;287:117331. doi: 10.1016/j.ecoenv.2024.117331. Epub 2024 Nov 14. Ecotoxicol Environ Saf. 2024. PMID: 39547060
-
Hemorrhagic shock augments Nlrp3 inflammasome activation in the lung through impaired pyrin induction.J Immunol. 2013 May 15;190(10):5247-55. doi: 10.4049/jimmunol.1203182. Epub 2013 Apr 12. J Immunol. 2013. PMID: 23585683 Free PMC article.
Cited by
-
Dopamine D1 receptor agonist A-68930 inhibits NLRP3 inflammasome activation and protects rats from spinal cord injury-induced acute lung injury.Spinal Cord. 2016 Nov;54(11):951-956. doi: 10.1038/sc.2016.52. Epub 2016 Apr 12. Spinal Cord. 2016. PMID: 27067657 Clinical Trial.
-
Can microRNAs keep inflammasomes in check?Front Genet. 2013 Mar 13;4:30. doi: 10.3389/fgene.2013.00030. eCollection 2013. Front Genet. 2013. PMID: 23495355 Free PMC article. No abstract available.
-
Iron and Sphingolipids as Common Players of (Mal)Adaptation to Hypoxia in Pulmonary Diseases.Int J Mol Sci. 2020 Jan 2;21(1):307. doi: 10.3390/ijms21010307. Int J Mol Sci. 2020. PMID: 31906427 Free PMC article. Review.
-
Toward the Development of Personalized Syndrome Discriminant Systems: A Discriminant System for Hypertension with Liver Yang Hyperactivity Syndrome.Evid Based Complement Alternat Med. 2021 Nov 15;2021:4532279. doi: 10.1155/2021/4532279. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 34819981 Free PMC article.
-
4-hydroxynonenal regulates mitochondrial function in human small airway epithelial cells.Oncotarget. 2015 Dec 8;6(39):41508-21. doi: 10.18632/oncotarget.6131. Oncotarget. 2015. PMID: 26484418 Free PMC article.
References
-
- Barnes PJ. Ceramide lances the lungs. Nat Med. 2004;10:130–131. - PubMed
-
- Goggel R, Winoto-Morbach S, Vielhaber G, Imai Y, Lindner K, Brade L, Brade H, Ehlers S, Slutsky AS, Schutze S, Gulbins E, Uhlig S. PAF-mediated pulmonary edema: a new role for acid sphingomyelinase and ceramide. Nat Med. 2004;10:155–160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

