Intersubunit bridge formation governs agonist efficacy at nicotinic acetylcholine α4β2 receptors: unique role of halogen bonding revealed
- PMID: 22170047
- PMCID: PMC3281707
- DOI: 10.1074/jbc.M111.292243
Intersubunit bridge formation governs agonist efficacy at nicotinic acetylcholine α4β2 receptors: unique role of halogen bonding revealed
Abstract
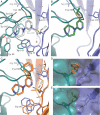
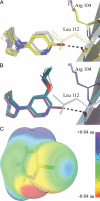
The α4β2 subtype of the nicotinic acetylcholine receptor has been pursued as a drug target for treatment of psychiatric and neurodegenerative disorders and smoking cessation aids for decades. Still, a thorough understanding of structure-function relationships of α4β2 agonists is lacking. Using binding experiments, electrophysiology and x-ray crystallography we have investigated a consecutive series of five prototypical pyridine-containing agonists derived from 1-(pyridin-3-yl)-1,4-diazepane. A correlation between binding affinities at α4β2 and the acetylcholine-binding protein from Lymnaea stagnalis (Ls-AChBP) confirms Ls-AChBP as structural surrogate for α4β2 receptors. Crystal structures of five agonists with efficacies at α4β2 from 21-76% were determined in complex with Ls-AChBP. No variation in closure of loop C is observed despite large efficacy variations. Instead, the efficacy of a compound appears tightly coupled to its ability to form a strong intersubunit bridge linking the primary and complementary binding interfaces. For the tested agonists, a specific halogen bond was observed to play a large role in establishing such strong intersubunit anchoring.
Figures
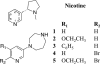
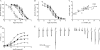

References
-
- Schneider J. S., Van Velson M., Menzaghi F., Lloyd G. K. (1998) Effects of the nicotinic acetylcholine receptor agonist SIB-1508Y on object retrieval performance in MPTP-treated monkeys. Comparison with levodopa treatment. Ann. Neurol. 43, 311–317 - PubMed
-
- Levin E. D., Christopher N. C., Weaver T., Moore J., Brucato F. (1999) Ventral hippocampal ibotenic acid lesions block chronic nicotine-induced spatial working memory improvement in rats. Brain Res. Cogn. Brain Res. 7, 405–410 - PubMed
-
- Steinlein O. K., Mulley J. C., Propping P., Wallace R. H., Phillips H. A., Sutherland G. R., Scheffer I. E., Berkovic S. F. (1995) A missense mutation in the neuronal nicotinic acetylcholine receptor α4 subunit is associated with autosomal dominant nocturnal frontal lobe epilepsy. Nat. Genet. 11, 201–203 - PubMed
-
- Grottick A. J., Higgins G. A. (2000) Effect of subtype selective nicotinic compounds on attention as assessed by the five-choice serial reaction time task. Behav Brain Res. 117, 197–208 - PubMed
-
- Jensen A. A., Frølund B., Liljefors T., Krogsgaard-Larsen P. (2005) Neuronal nicotinic acetylcholine receptors. Structural revelations, target identifications, and therapeutic inspirations. J. Med. Chem. 48, 4705–4745 - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Chemical Information

