Glutathione degradation is a key determinant of glutathione homeostasis
- PMID: 22170048
- PMCID: PMC3281641
- DOI: 10.1074/jbc.M111.315705
Glutathione degradation is a key determinant of glutathione homeostasis
Abstract
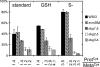
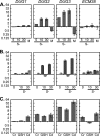
Glutathione (GSH) has several important functions in eukaryotic cells, and its intracellular concentration is tightly controlled. Combining mathematical models and (35)S labeling, we analyzed Saccharomyces cerevisiae sulfur metabolism. This led us to the observation that GSH recycling is markedly faster than previously estimated. We set up additional in vivo assays and concluded that under standard conditions, GSH half-life is around 90 min. Sulfur starvation and growth with GSH as the sole sulfur source strongly increase GSH degradation, whereas cadmium (Cd(2+)) treatment inhibits GSH degradation. Whatever the condition tested, GSH is degraded by the cytosolic Dug complex (composed of the three subunits Dug1, Dug2, and Dug3) but not by the γ-glutamyl-transpeptidase, raising the question of the role of this enzyme. In vivo, both DUG2/3 mRNA levels and Dug activity are quickly induced by sulfur deprivation in a Met4-dependent manner. This suggests that Dug activity is mainly regulated at the transcriptional level. Finally, analysis of dug2Δ and dug3Δ mutant cells shows that GSH degradation activity strongly impacts on GSH intracellular concentration and that GSH intracellular concentration does not affect GSH synthesis rate. Altogether, our data led us to reconsider important aspects of GSH metabolism, challenging notions on GSH synthesis and GSH degradation that were considered as established.
Figures





References
-
- Lu S. C. (1999) Regulation of hepatic glutathione synthesis. Current concepts and controversies. FASEB J. 13, 1169–1183 - PubMed
-
- Bourbouloux A., Shahi P., Chakladar A., Delrot S., Bachhawat A. K. (2000) Hgt1p, a high affinity glutathione transporter from the yeast Saccharomyces cerevisiae. J. Biol. Chem. 275, 13259–13265 - PubMed
-
- Rebbeor J. F., Connolly G. C., Dumont M. E., Ballatori N. (1998) ATP-dependent transport of reduced glutathione on YCF1, the yeast orthologue of mammalian multidrug resistance- associated proteins. J. Biol. Chem. 273, 33449–33454 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

