Cytosolic carboxypeptidase 1 is involved in processing α- and β-tubulin
- PMID: 22170066
- PMCID: PMC3307270
- DOI: 10.1074/jbc.M111.309138
Cytosolic carboxypeptidase 1 is involved in processing α- and β-tubulin
Abstract
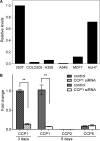
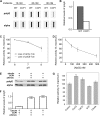
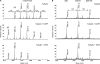
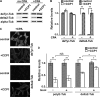
The Purkinje cell degeneration (pcd) mouse has a disruption in the gene encoding cytosolic carboxypeptidase 1 (CCP1). This study tested two proposed functions of CCP1: degradation of intracellular peptides and processing of tubulin. Overexpression (2-3-fold) or knockdown (80-90%) of CCP1 in human embryonic kidney 293T cells (HEK293T) did not affect the levels of most intracellular peptides but altered the levels of α-tubulin lacking two C-terminal amino acids (delta2-tubulin) ≥ 5-fold, suggesting that tubulin processing is the primary function of CCP1, not peptide degradation. Purified CCP1 produced delta2-tubulin from purified porcine brain α-tubulin or polymerized HEK293T microtubules. In addition, CCP1 removed Glu residues from the polyglutamyl side chains of porcine brain α- and β-tubulin and also generated a form of α-tubulin with two C-terminal Glu residues removed (delta3-tubulin). Consistent with this, pcd mouse brain showed hyperglutamylation of both α- and β-tubulin. The hyperglutamylation of α- and β-tubulin and subsequent death of Purkinje cells in pcd mice was counteracted by the knock-out of the gene encoding tubulin tyrosine ligase-like-1, indicating that this enzyme hyperglutamylates α- and β-tubulin. Taken together, these results demonstrate a role for CCP1 in the processing of Glu residues from β- as well as α-tubulin in vitro and in vivo.
Figures



References
-
- Harris A., Morgan J. I., Pecot M., Soumare A., Osborne A., Soares H. D. (2000) Regenerating motor neurons express Nna1, a novel ATP/GTP-binding protein related to zinc carboxypeptidases. Mol. Cell. Neurosci. 16, 578–596 - PubMed
-
- Kalinina E., Biswas R., Berezniuk I., Hermoso A., Aviles F. X., Fricker L. D. (2007) A novel subfamily of mouse cytosolic carboxypeptidases. FASEB J. 21, 836–850 - PubMed
-
- Rodriguez de la Vega M., Sevilla R. G., Hermoso A., Lorenzo J., Tanco S., Diez A., Fricker L. D., Bautista J. M., Avilés F. X. (2007) Nna1-like proteins are active metallocarboxypeptidases of a new and diverse M14 subfamily. FASEB J. 21, 851–865 - PubMed
-
- Fernandez-Gonzalez A., La Spada A. R., Treadaway J., Higdon J. C., Harris B. S., Sidman R. L., Morgan J. I., Zuo J. (2002) Purkinje cell degeneration (pcd) phenotypes caused by mutations in the axotomy-induced gene, Nna1. Science 295, 1904–1906 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

