A novel fortified blended flour, corn-soy blend "plus-plus," is not inferior to lipid-based ready-to-use supplementary foods for the treatment of moderate acute malnutrition in Malawian children
- PMID: 22170366
- PMCID: PMC3238461
- DOI: 10.3945/ajcn.111.022525
A novel fortified blended flour, corn-soy blend "plus-plus," is not inferior to lipid-based ready-to-use supplementary foods for the treatment of moderate acute malnutrition in Malawian children
Abstract
Background: Children with moderate acute malnutrition (MAM) are often treated with fortified blended flours, most commonly a corn-soy blend (CSB). However, recovery rates remain <75%, lower than the rate achieved with peanut paste-based ready-to-use supplementary foods (RUSFs). To bridge this gap, a novel CSB recipe fortified with oil and dry skim milk, "CSB++," has been developed.
Objective: In this trial we compared CSB++ with 2 RUSF products for the treatment of MAM to test the hypothesis that the recovery rate achieved with CSB++ will not be >5% worse than that achieved with either RUSF.
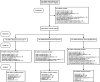
Design: We conducted a prospective, randomized, investigator-blinded, controlled noninferiority trial involving rural Malawian children aged 6-59 mo with MAM. Children received 75 kcal CSB++ · kg(-1) · d(-1), locally produced soy RUSF, or an imported soy/whey RUSF for ≤12 wk.
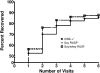
Results: The recovery rate for CSB++ (n = 763 of 888; 85.9%) was similar to that for soy RUSF (795 of 806, 87.7%; risk difference: -1.82%; 95% CI: -4.95%, 1.30%) and soy/whey RUSF (807 of 918, 87.9%; risk difference: -1.99%; 95% CI: -5.10%, 1.13%). On average, children who received CSB++ required 2 d longer to recover, and the rate of weight gain was less than that with either RUSF, although height gain was the same among all 3 foods studied.
Conclusions: A novel, locally produced, fortified blended flour (CSB++) was not inferior to a locally produced soy RUSF and an imported soy/whey RUSF in facilitating recovery from MAM. The recovery rate observed for CSB++ was higher than that for any other fortified blended flour tested previously. This trial is registered at clinicaltrials.gov as NCT00998517.
Figures
Similar articles
-
Including whey protein and whey permeate in ready-to-use supplementary food improves recovery rates in children with moderate acute malnutrition: a randomized, double-blind clinical trial.Am J Clin Nutr. 2016 Mar;103(3):926-33. doi: 10.3945/ajcn.115.124636. Epub 2016 Feb 10. Am J Clin Nutr. 2016. PMID: 26864368 Clinical Trial.
-
Recovery rate of children with moderate acute malnutrition treated with ready-to-use supplementary food (RUSF) or improved corn-soya blend (CSB+): a randomized controlled trial.Public Health Nutr. 2016 Feb;19(2):363-70. doi: 10.1017/S1368980015001238. Epub 2015 May 5. Public Health Nutr. 2016. PMID: 25939394 Free PMC article. Clinical Trial.
-
Effectiveness and cost-effectiveness of 4 supplementary foods for treating moderate acute malnutrition: results from a cluster-randomized intervention trial in Sierra Leone.Am J Clin Nutr. 2021 Sep 1;114(3):973-985. doi: 10.1093/ajcn/nqab140. Am J Clin Nutr. 2021. PMID: 34020452 Free PMC article. Clinical Trial.
-
Effectiveness of interventions to manage acute malnutrition in children under 5 years of age in low- and middle-income countries: A systematic review.Campbell Syst Rev. 2020 Apr 9;16(2):e1082. doi: 10.1002/cl2.1082. eCollection 2020 Jun. Campbell Syst Rev. 2020. PMID: 37131422 Free PMC article. Review.
-
Current evidence on the effectiveness of Ready-to-Use Supplementary Foods in children with moderate acute malnutrition: a systematic review and meta-analysis.J Nutr Sci. 2024 Jan 3;12:e130. doi: 10.1017/jns.2023.114. eCollection 2023. J Nutr Sci. 2024. PMID: 38179261 Free PMC article.
Cited by
-
Time to Recovery From Moderate Acute Malnutrition and Its Predictors Among Children 6-59 Months of Age Enrolled in Targeted Supplementary Feeding Program in Darolebu District, Eastern Ethiopia: A Retrospective Cohort Study.Front Public Health. 2022 Jul 14;10:914837. doi: 10.3389/fpubh.2022.914837. eCollection 2022. Front Public Health. 2022. PMID: 35910899 Free PMC article.
-
Acceptability of locally-produced Ready-to-Use Supplementary Food (RUSF) for children under two years in Cambodia: A cluster randomised trial.Matern Child Nutr. 2019 Jul;15(3):e12780. doi: 10.1111/mcn.12780. Epub 2019 Feb 20. Matern Child Nutr. 2019. PMID: 30690869 Free PMC article. Clinical Trial.
-
An emergency cash transfer program promotes weight gain and reduces acute malnutrition risk among children 6-24 months old during a food crisis in Niger.J Glob Health. 2018 Jun;8(1):010410. doi: 10.7189/jogh.08.010410. J Glob Health. 2018. PMID: 29497505 Free PMC article.
-
Wasting coexisting with underweight and stunting among children aged 6‒59 months hospitalised in Garissa County Referral Hospital, Kenya.Matern Child Nutr. 2025 Jan;21(1):e13754. doi: 10.1111/mcn.13754. Epub 2024 Oct 24. Matern Child Nutr. 2025. PMID: 39449066 Free PMC article.
-
Ready-to-use therapeutic food (RUTF) for home-based nutritional rehabilitation of severe acute malnutrition in children from six months to five years of age.Cochrane Database Syst Rev. 2019 May 15;5(5):CD009000. doi: 10.1002/14651858.CD009000.pub3. Cochrane Database Syst Rev. 2019. PMID: 31090070 Free PMC article.
References
-
- Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 2008;371:243–60 - PubMed
-
- Shankar AH. Nutritional modulation of malaria morbidity and mortality. J Infect Dis 2000;182(suppl 1):S37–53 - PubMed
-
- Berkman DS, Lescano AG, Gilman RH, Lopez SL, Black MM. Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: a follow-up study. Lancet 2002;359:564–71 - PubMed
-
- Fishman SCL, de Onis M, Blossner M, Mullany L, Black RE. Malnutrition and the global burden of disease: underweight. Cambridge, MA: World Health Organization/Harvard University Press, 2003
-
- Marchione TJ. Foods provided through U.S. Government Emergency Food Aid Programs: policies and customs governing their formulation, selection and distribution. J Nutr 2002;132:2104S–11S - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

