Aldo-keto reductase 1C3 is expressed in differentiated human epidermis, affects keratinocyte differentiation, and is upregulated in atopic dermatitis
- PMID: 22170488
- PMCID: PMC3305848
- DOI: 10.1038/jid.2011.412
Aldo-keto reductase 1C3 is expressed in differentiated human epidermis, affects keratinocyte differentiation, and is upregulated in atopic dermatitis
Abstract
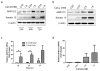
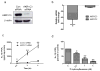
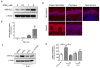
Aldo-keto reductase 1C3 (AKR1C3) has been shown to mediate the metabolism of sex hormones and prostaglandin D(2) (PGD(2)), a lipid mediator that promotes skin inflammation in atopic dermatitis (AD). As both have a role in skin function and pathology, we first sought to investigate the expression pattern of AKR1C3 in normal human epidermis. Immunofluorescence revealed a strong expression of AKR1C3 in the differentiated suprabasal layers compared with the basal layer. Western blot analysis and quantitative PCR confirmed that AKR1C3 expression was also upregulated in differentiation-induced primary human keratinocytes (PHKs). To investigate the functional role of AKR1C3 during PHK differentiation, its expression and activity (measured as PGD(2) reduction to 9α,11β-PGF(2) by ELISA) were impaired by small interfering RNA or 2'-hydroxyflavanone, respectively. Cytokeratin 10 (K10) and loricrin expression were then examined by western blot analysis, thus revealing altered expression of these differentiation markers. Finally, following an observation that the AD-associated mediator, PGD(2), upregulated AKR1C3 expression in PHKs, we used immunofluorescence to examine AKR1C3 expression in AD and psoriasis lesions. AKR1C3 was found to be upregulated in AD but not in psoriasis lesions compared with non-lesional skin. Our work demonstrates a function for AKR1C3 in differentiation-associated gene regulation and also suggests a role in supporting inflammation in AD.
Conflict of interest statement
The authors state no conflict of interest.
Figures

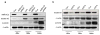
Similar articles
-
Aldo-keto reductase 1C3 expression in MCF-7 cells reveals roles in steroid hormone and prostaglandin metabolism that may explain its over-expression in breast cancer.J Steroid Biochem Mol Biol. 2010 Feb 15;118(3):177-87. doi: 10.1016/j.jsbmb.2009.12.009. Epub 2009 Dec 28. J Steroid Biochem Mol Biol. 2010. PMID: 20036328 Free PMC article.
-
Aldo-keto reductase 1C3 is overexpressed in skin squamous cell carcinoma (SCC) and affects SCC growth via prostaglandin metabolism.Exp Dermatol. 2014 Aug;23(8):573-8. doi: 10.1111/exd.12468. Epub 2014 Jul 16. Exp Dermatol. 2014. PMID: 24917395 Free PMC article.
-
The role of aldo-keto reductase 1C3 (AKR1C3)-mediated prostaglandin D2 (PGD2) metabolism in keloids.Exp Dermatol. 2016 Jan;25(1):38-43. doi: 10.1111/exd.12854. Epub 2015 Oct 6. Exp Dermatol. 2016. PMID: 26308156
-
Inhibitors of type 5 17β-hydroxysteroid dehydrogenase (AKR1C3): overview and structural insights.J Steroid Biochem Mol Biol. 2011 May;125(1-2):95-104. doi: 10.1016/j.jsbmb.2010.11.004. Epub 2010 Nov 16. J Steroid Biochem Mol Biol. 2011. PMID: 21087665 Free PMC article. Review.
-
AKR1C3 as a target in castrate resistant prostate cancer.J Steroid Biochem Mol Biol. 2013 Sep;137:136-49. doi: 10.1016/j.jsbmb.2013.05.012. Epub 2013 Jun 6. J Steroid Biochem Mol Biol. 2013. PMID: 23748150 Free PMC article. Review.
Cited by
-
Knockdown of AKR1C3 exposes a potential epigenetic susceptibility in prostate cancer cells.J Steroid Biochem Mol Biol. 2016 Jan;155(Pt A):47-55. doi: 10.1016/j.jsbmb.2015.09.037. Epub 2015 Sep 30. J Steroid Biochem Mol Biol. 2016. PMID: 26429394 Free PMC article.
-
Atopic Dermatitis: The Relationship Between Immune Mediators and Skin Lipid Barrier.Clin Rev Allergy Immunol. 2025 May 14;68(1):49. doi: 10.1007/s12016-025-09057-y. Clin Rev Allergy Immunol. 2025. PMID: 40366491 Review.
-
Development of Human Adrenocortical Adenoma (HAA1) Cell Line from Zona Reticularis.Int J Mol Sci. 2022 Dec 29;24(1):584. doi: 10.3390/ijms24010584. Int J Mol Sci. 2022. PMID: 36614027 Free PMC article.
-
Identification of meibomian gland testosterone metabolites produced by tissue-intrinsic intracrine deactivation activity.iScience. 2025 Jan 27;28(2):111808. doi: 10.1016/j.isci.2025.111808. eCollection 2025 Feb 21. iScience. 2025. PMID: 39995859 Free PMC article.
-
Human AKR1C3 binds agonists of GPR84 and participates in an expanded polyamine pathway.Cell Chem Biol. 2025 Jan 16;32(1):126-144.e18. doi: 10.1016/j.chembiol.2024.07.011. Epub 2024 Aug 19. Cell Chem Biol. 2025. PMID: 39163853
References
-
- Dahten A, Koch C, Ernst D, Schnoller C, Hartmann S, Worm M. Systemic PPARgamma ligation inhibits allergic immune response in the skin. J Invest Dermatol. 2008;128:2211–8. - PubMed
-
- Desmond JC, Mountford JC, Drayson MT, Walker EA, Hewison M, Ride JP, et al. The aldo-keto reductase AKR1C3 is a novel suppressor of cell differentiation that provides a plausible target for the non-cyclooxygenase-dependent antineoplastic actions of nonsteroidal anti-inflammatory drugs. Cancer Res. 2003;63:505–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

