IκB kinase epsilon (IKK(epsilon)) regulates the balance between type I and type II interferon responses
- PMID: 22171011
- PMCID: PMC3248534
- DOI: 10.1073/pnas.1119137109
IκB kinase epsilon (IKK(epsilon)) regulates the balance between type I and type II interferon responses
Abstract
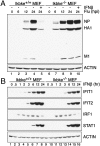
Virus infection induces the production of type I and type II interferons (IFN-I and IFN-II), cytokines that mediate the antiviral response. IFN-I (IFN-α and IFN-β) induces the assembly of IFN-stimulated gene factor 3 (ISGF3), a multimeric transcriptional activation complex composed of STAT1, STAT2, and IFN regulatory factor 9. IFN-II (IFN-γ) induces the homodimerization of STAT1 to form the gamma-activated factor (GAF) complex. ISGF3 and GAF bind specifically to unique regulatory DNA sequences located upstream of IFN-I- and IFN-II-inducible genes, respectively, and activate the expression of distinct sets of antiviral genes. The balance between type I and type II IFN pathways plays a critical role in orchestrating the innate and adaptive immune systems. Here, we show that the phosphorylation of STAT1 by IκB kinase epsilon (IKKε) inhibits STAT1 homodimerization, and thus assembly of GAF, but does not disrupt ISGF3 formation. Therefore, virus and/or IFN-I activation of IKKε suppresses GAF-dependent transcription and promotes ISGF3-dependent transcription. In the absence of IKKε, GAF-dependent transcription is enhanced at the expense of ISGF3-mediated transcription, rendering cells less resistant to infection. We conclude that IKKε plays a critical role in regulating the balance between the IFN-I and IFN-II signaling pathways.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
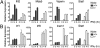



References
-
- Thompson AJ, Locarnini SA. Toll-like receptors, RIG-I-like RNA helicases and the antiviral innate immune response. Immunol Cell Biol. 2007;85:435–445. - PubMed
-
- Baumgarth N, Choi YS, Rothaeusler K, Yang Y, Herzenberg LA. B cell lineage contributions to antiviral host responses. Curr Top Microbiol Immunol. 2008;319:41–61. - PubMed
-
- Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5:375–386. - PubMed
-
- Hardy MP, Owczarek CM, Jermiin LS, Ejdebäck M, Hertzog PJ. Characterization of the type I interferon locus and identification of novel genes. Genomics. 2004;84:331–345. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

