Neurosteroidogenesis is required for the physiological response to stress: role of neurosteroid-sensitive GABAA receptors
- PMID: 22171026
- PMCID: PMC3272883
- DOI: 10.1523/JNEUROSCI.2560-11.2011
Neurosteroidogenesis is required for the physiological response to stress: role of neurosteroid-sensitive GABAA receptors
Abstract
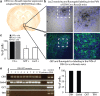
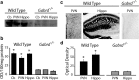
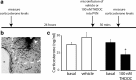
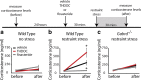
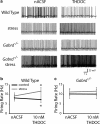
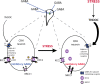
The hypothalamic-pituitary-adrenal (HPA) axis, which mediates the body's response to stress, is largely under GABAergic control. Here we demonstrate that corticotropin-releasing hormone (CRH) neurons are modulated by the stress-derived neurosteroid, tetrahydrodeoxycorticosterone (THDOC), acting on δ subunit-containing GABA(A) receptors (GABA(A)Rs). Under normal conditions, THDOC potentiates the inhibitory effects of GABA on CRH neurons, decreasing the activity of the HPA axis. Counterintuitively, following stress, THDOC activates the HPA axis due to dephosphorylation of KCC2 residue Ser940, resulting in a collapse of the chloride gradient and excitatory GABAergic transmission. The effects of THDOC on CRH neurons are mediated by actions on GABA(A)R δ subunit-containing receptors since these effects are abolished in Gabrd(-/-) mice under both control and stress conditions. Interestingly, blocking neurosteroidogenesis with finasteride is sufficient to block the stress-induced elevations in corticosterone and prevent stress-induced anxiety-like behaviors in mice. These data demonstrate that positive feedback of neurosteroids onto CRH neurons is required to mount the physiological response to stress. Further, GABA(A)R δ subunit-containing receptors and phosphorylation of KCC2 residue Ser940 may be novel targets for control of the stress response, which has therapeutic potential for numerous disorders associated with hyperexcitability of the HPA axis, including Cushing's syndrome, epilepsy, and major depression.
Figures





Similar articles
-
Seizure-induced activation of the HPA axis increases seizure frequency and comorbid depression-like behaviors.Epilepsy Behav. 2018 Jan;78:124-133. doi: 10.1016/j.yebeh.2017.10.025. Epub 2017 Dec 22. Epilepsy Behav. 2018. PMID: 29186699 Free PMC article.
-
GABAergic regulation of the HPA and HPG axes and the impact of stress on reproductive function.J Steroid Biochem Mol Biol. 2016 Jun;160:196-203. doi: 10.1016/j.jsbmb.2015.11.019. Epub 2015 Dec 9. J Steroid Biochem Mol Biol. 2016. PMID: 26690789 Free PMC article. Review.
-
Loss of Gabrd in CRH neurons blunts the corticosterone response to stress and diminishes stress-related behaviors.Psychoneuroendocrinology. 2014 Mar;41:75-88. doi: 10.1016/j.psyneuen.2013.12.011. Epub 2013 Dec 24. Psychoneuroendocrinology. 2014. PMID: 24495609 Free PMC article.
-
Inability to suppress the stress-induced activation of the HPA axis during the peripartum period engenders deficits in postpartum behaviors in mice.Psychoneuroendocrinology. 2018 Apr;90:182-193. doi: 10.1016/j.psyneuen.2017.12.003. Epub 2017 Dec 21. Psychoneuroendocrinology. 2018. PMID: 29274662 Free PMC article.
-
Neurosteroids; potential underpinning roles in maintaining homeostasis.Gen Comp Endocrinol. 2016 Jan 1;225:242-250. doi: 10.1016/j.ygcen.2015.09.030. Epub 2015 Oct 21. Gen Comp Endocrinol. 2016. PMID: 26432100 Review.
Cited by
-
Environmental regulation of the chloride transporter KCC2: switching inflammation off to switch the GABA on?Transl Psychiatry. 2020 Oct 15;10(1):349. doi: 10.1038/s41398-020-01027-6. Transl Psychiatry. 2020. PMID: 33060559 Free PMC article. Review.
-
Neurosteroids increase tonic GABAergic inhibition in the lateral section of the central amygdala in mice.J Neurophysiol. 2015 May 1;113(9):3421-31. doi: 10.1152/jn.00045.2015. Epub 2015 Mar 18. J Neurophysiol. 2015. PMID: 25787948 Free PMC article.
-
Stress: Influence of sex, reproductive status and gender.Neurobiol Stress. 2019 Mar 9;10:100155. doi: 10.1016/j.ynstr.2019.100155. eCollection 2019 Feb. Neurobiol Stress. 2019. PMID: 30949564 Free PMC article. Review.
-
(3α,5α)3-Hydroxypregnan-20-one (3α,5α-THP) Regulation of the HPA Axis in the Context of Different Stressors and Sex.Biomolecules. 2022 Aug 18;12(8):1134. doi: 10.3390/biom12081134. Biomolecules. 2022. PMID: 36009028 Free PMC article.
-
Female mice with deletion of Type One 5α-reductase have reduced reproductive responding during proestrus and after hormone-priming.Pharmacol Biochem Behav. 2014 Jul;122:20-9. doi: 10.1016/j.pbb.2014.03.010. Epub 2014 Mar 18. Pharmacol Biochem Behav. 2014. PMID: 24650589 Free PMC article.
References
-
- Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International Union of Pharmacology. XV. Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. - PubMed
-
- Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABA(A) receptors. Neuropharmacology. 2002;43:651–661. - PubMed
-
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. - PubMed
-
- Boehm SL, 2nd, Homanics GE, Blednov YA, Harris RA. delta-Subunit GABAA receptor knockout mice are less sensitive to the actions of 4,5,6,7-tetrahydroisoxazolo-[5,4-c]pyridin-3-ol. Eur J Pharmacol. 2006;541:158–162. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
