Intrinsic reference frames of superior colliculus visuomotor receptive fields during head-unrestrained gaze shifts
- PMID: 22171035
- PMCID: PMC6623887
- DOI: 10.1523/JNEUROSCI.0990-11.2011
Intrinsic reference frames of superior colliculus visuomotor receptive fields during head-unrestrained gaze shifts
Abstract
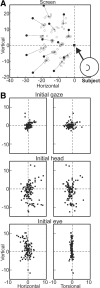
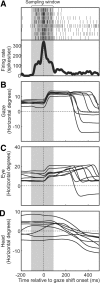
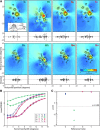
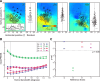
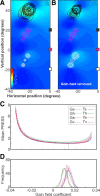
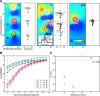
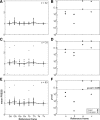
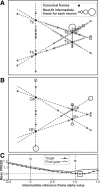
A sensorimotor neuron's receptive field and its frame of reference are easily conflated within the natural variability of spatial behavior. Here, we capitalized on such natural variations in 3-D eye and head positions during head-unrestrained gaze shifts to visual targets in two monkeys: to determine whether intermediate/deep layer superior colliculus (SC) receptive fields code visual targets or gaze kinematics, within four different frames of reference. Visuomotor receptive fields were either characterized during gaze shifts to visual targets from a central fixation position (32 U) or were partially characterized from each of three initial fixation points (31 U). Natural variations of initial 3-D gaze and head orientation (including torsion) provided spatial separation between four different coordinate frame models (space, head, eye, fixed-vector relative to fixation), whereas natural saccade errors provided spatial separation between target and gaze positions. Using a new statistical method based on predictive sum-of-squares, we found that in our population of 63 neurons (1) receptive field fits to target positions were significantly better than fits to actual gaze shift locations and (2) eye-centered models gave significantly better fits than the head or space frame. An intermediate frames analysis confirmed that individual neuron fits were distributed target-in-eye coordinates. Gaze position "gain" effects with the spatial tuning required for a 3-D reference frame transformation were significant in 23% (7/31) of neurons tested. We conclude that the SC primarily represents gaze targets relative to the eye but also carries early signatures of the 3-D sensorimotor transformation.
Figures


Similar articles
-
Spatiotemporal transformations for gaze control.Physiol Rep. 2020 Aug;8(16):e14533. doi: 10.14814/phy2.14533. Physiol Rep. 2020. PMID: 32812395 Free PMC article. Review.
-
Spatial transformations between superior colliculus visual and motor response fields during head-unrestrained gaze shifts.Eur J Neurosci. 2015 Dec;42(11):2934-51. doi: 10.1111/ejn.13093. Eur J Neurosci. 2015. PMID: 26448341
-
Beyond the labeled line: variation in visual reference frames from intraparietal cortex to frontal eye fields and the superior colliculus.J Neurophysiol. 2018 Apr 1;119(4):1411-1421. doi: 10.1152/jn.00584.2017. Epub 2017 Dec 20. J Neurophysiol. 2018. PMID: 29357464 Free PMC article.
-
Saccades to somatosensory targets. III. eye-position-dependent somatosensory activity in primate superior colliculus.J Neurophysiol. 1996 Jan;75(1):439-53. doi: 10.1152/jn.1996.75.1.439. J Neurophysiol. 1996. PMID: 8822569
-
Shifts in the eye-centered frame of reference may underlie saccades, visual perception, and eye-hand coordination.J Neurophysiol. 2022 Oct 1;128(4):1025-1039. doi: 10.1152/jn.00531.2021. Epub 2022 Sep 7. J Neurophysiol. 2022. PMID: 36070246 Review.
Cited by
-
Timing Determines Tuning: A Rapid Spatial Transformation in Superior Colliculus Neurons during Reactive Gaze Shifts.eNeuro. 2020 Jan 3;7(1):ENEURO.0359-18.2019. doi: 10.1523/ENEURO.0359-18.2019. Print 2020 Jan/Feb. eNeuro. 2020. PMID: 31792117 Free PMC article.
-
Transition from Target to Gaze Coding in Primate Frontal Eye Field during Memory Delay and Memory-Motor Transformation.eNeuro. 2016 Apr 13;3(2):ENEURO.0040-16.2016. doi: 10.1523/ENEURO.0040-16.2016. eCollection 2016 Mar-Apr. eNeuro. 2016. PMID: 27092335 Free PMC article.
-
Multiunit Frontal Eye Field Activity Codes the Visuomotor Transformation, But Not Gaze Prediction or Retrospective Target Memory, in a Delayed Saccade Task.eNeuro. 2024 Aug 8;11(8):ENEURO.0413-23.2024. doi: 10.1523/ENEURO.0413-23.2024. Print 2024 Aug. eNeuro. 2024. PMID: 39054056 Free PMC article.
-
Spatiotemporal transformations for gaze control.Physiol Rep. 2020 Aug;8(16):e14533. doi: 10.14814/phy2.14533. Physiol Rep. 2020. PMID: 32812395 Free PMC article. Review.
-
Spatial localization of sound elicits early responses from occipital visual cortex in humans.Sci Rep. 2017 Sep 5;7(1):10415. doi: 10.1038/s41598-017-09142-z. Sci Rep. 2017. PMID: 28874681 Free PMC article.
References
-
- Andersen RA, Essick GK, Siegel RM. Encoding of spatial location by posterior parietal neurons. Science. 1985;230:456–458. - PubMed
-
- Avillac M, Denève S, Olivier E, Pouget A, Duhamel JR. Reference frames for representing visual and tactile locations in parietal cortex. Nat Neurosci. 2005;8:941–949. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
