Role of the glyoxalase system in astrocyte-mediated neuroprotection
- PMID: 22171037
- PMCID: PMC6623908
- DOI: 10.1523/JNEUROSCI.1249-11.2011
Role of the glyoxalase system in astrocyte-mediated neuroprotection
Abstract
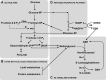
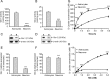
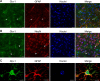
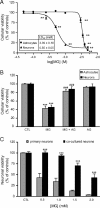
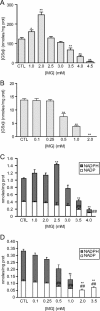
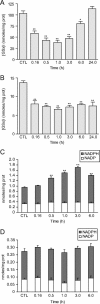
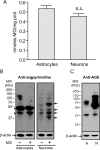
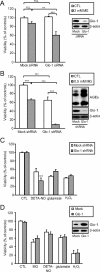
The glyoxalase system is the most important pathway for the detoxification of methylglyoxal (MG), a highly reactive dicarbonyl compound mainly formed as a by-product of glycolysis. MG is a major precursor of advanced glycation end products (AGEs), which are associated with several neurodegenerative disorders. Although the neurotoxic effects of MG and AGEs are well characterized, little is known about the glyoxalase system in the brain, in particular with regards to its activity in different neural cell types. Results of the present study reveal that both enzymes composing the glyoxalase system [glyoxalase-1 (Glo-1) and Glo-2] were highly expressed in primary mouse astrocytes compared with neurons, which translated into higher enzymatic activity rates in astrocytes (9.9- and 2.5-fold, respectively). The presence of a highly efficient glyoxalase system in astrocytes was associated with lower accumulation of AGEs compared with neurons (as assessed by Western blotting), a sixfold greater resistance to MG toxicity, and the capacity to protect neurons against MG in a coculture system. In addition, Glo-1 downregulation using RNA interference strategies resulted in a loss of viability in neurons, but not in astrocytes. Finally, stimulation of neuronal glycolysis via lentiviral-mediated overexpression of 6-phosphofructose-2-kinase/fructose-2,6-bisphosphatase-3 resulted in increased MG levels and MG-modified proteins. Since MG is largely produced through glycolysis, this suggests that the poor capacity of neurons to upregulate their glycolytic flux as compared with astrocytes may be related to weaker defense mechanisms against MG toxicity. Accordingly, the neuroenergetic specialization taking place between these two cell types may serve as a protective mechanism against MG-induced neurotoxicity.
Figures
Similar articles
-
Tat-glyoxalase protein inhibits against ischemic neuronal cell damage and ameliorates ischemic injury.Free Radic Biol Med. 2014 Feb;67:195-210. doi: 10.1016/j.freeradbiomed.2013.10.815. Epub 2013 Nov 16. Free Radic Biol Med. 2014. PMID: 24252591
-
Methylglyoxal, the dark side of glycolysis.Front Neurosci. 2015 Feb 9;9:23. doi: 10.3389/fnins.2015.00023. eCollection 2015. Front Neurosci. 2015. PMID: 25709564 Free PMC article. Review.
-
Flavonoid Enhances the Glyoxalase Pathway in Cerebellar Neurons to Retain Cellular Functions.Sci Rep. 2017 Jul 11;7(1):5126. doi: 10.1038/s41598-017-05287-z. Sci Rep. 2017. PMID: 28698611 Free PMC article.
-
Age-dependent changes of glyoxalase I expression in human brain.Neurobiol Aging. 2006 Jun;27(6):815-22. doi: 10.1016/j.neurobiolaging.2005.04.006. Epub 2005 Jun 9. Neurobiol Aging. 2006. PMID: 15950319
-
Glyoxalase System in the Progression of Skin Aging and Skin Malignancies.Int J Mol Sci. 2020 Dec 30;22(1):310. doi: 10.3390/ijms22010310. Int J Mol Sci. 2020. PMID: 33396745 Free PMC article. Review.
Cited by
-
Methylglyoxal, the foe and friend of glyoxalase and Trx/TrxR systems in HT22 nerve cells.Free Radic Biol Med. 2015 Dec;89:8-19. doi: 10.1016/j.freeradbiomed.2015.07.005. Epub 2015 Jul 9. Free Radic Biol Med. 2015. PMID: 26165190 Free PMC article.
-
Temporal dynamics of glyoxalase 1 in secondary neuronal injury.PLoS One. 2014 Feb 3;9(2):e87364. doi: 10.1371/journal.pone.0087364. eCollection 2014. PLoS One. 2014. PMID: 24498315 Free PMC article.
-
Is Methylglyoxal a Potential Biomarker for the Warburg Effect Induced by the Lipopolysaccharide Neuroinflammation Model?Neurochem Res. 2024 Jul;49(7):1823-1837. doi: 10.1007/s11064-024-04142-8. Epub 2024 May 10. Neurochem Res. 2024. PMID: 38727985
-
RAGE and glyoxalase in kidney disease.Glycoconj J. 2016 Aug;33(4):619-26. doi: 10.1007/s10719-016-9689-8. Epub 2016 Jun 6. Glycoconj J. 2016. PMID: 27270765 Review.
-
The Astrocyte: Powerhouse and Recycling Center.Cold Spring Harb Perspect Biol. 2015 Feb 13;7(12):a020396. doi: 10.1101/cshperspect.a020396. Cold Spring Harb Perspect Biol. 2015. PMID: 25680832 Free PMC article. Review.
References
-
- Ahmed N, Battah S, Karachalias N, Babaei-Jadidi R, Horányi M, Baróti K, Hollan S, Thornalley PJ. Increased formation of methylglyoxal and protein glycation, oxidation and nitrosation in triosephosphate isomerase deficiency. Biochim Biophys Acta. 2003;1639:121–132. - PubMed
-
- Allaman I, Pellerin L, Magistretti PJ. Glucocorticoids modulate neurotransmitter-induced glycogen metabolism in cultured cortical astrocytes. J Neurochem. 2004;88:900–908. - PubMed
-
- Allaman I, Bélanger M, Magistretti PJ. Astrocyte-neuron metabolic relationships: for better and for worse. Trends Neurosci. 2011;34:76–87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
