Bidirectional ephrinB3/EphA4 signaling mediates the segregation of medial ganglionic eminence- and preoptic area-derived interneurons in the deep and superficial migratory stream
- PMID: 22171039
- PMCID: PMC6623906
- DOI: 10.1523/JNEUROSCI.4690-11.2011
Bidirectional ephrinB3/EphA4 signaling mediates the segregation of medial ganglionic eminence- and preoptic area-derived interneurons in the deep and superficial migratory stream
Abstract
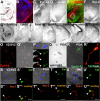
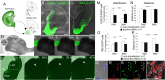
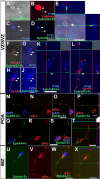
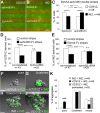
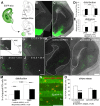
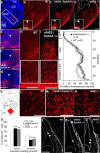
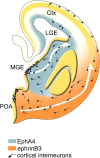
The integration of interneuron subtypes into specific microcircuits is essential for proper cortical function. Understanding to what extent interneuron diversity is regulated and maintained during development might help to reveal the principles that govern their role as synchronizing elements as well as causes for dysfunction. Particular interneuron subtypes are generated in a temporally regulated manner in the medial ganglionic eminence (MGE), the caudal ganglionic eminence, and the preoptic area (POA) of the basal telencephalon. Long-range tangential migration from their site of origin to cortical targets is orchestrated by a variety of attractive, repulsive, membrane-bound, and secreted signaling molecules, to establish the critical balance of inhibition and excitation. It remains unknown whether interneurons deriving from distinct domains are predetermined to migrate in particular routes and whether this process underlies cell type-specific regulation. We found that POA- and MGE-derived cortical interneurons migrate within spatially segregated corridors. EphrinB3, expressed in POA-derived interneurons traversing the superficial route, acts as a repellent signal for deeply migrating interneurons born in the MGE, which is mediated by EphA4 forward signaling. In contrast, EphA4 induces repulsive ephrinB3 reverse signaling in interneurons generated in the POA, restricting this population to the superficial path. Perturbation of this bidirectional ephrinB3/EphA4 signaling in vitro and in vivo leads to a partial intermingling of cells in these segregated migratory pathways. Thus, we conclude that cell contact-mediated bidirectional ephrinB3/EphA4 signaling mediates the sorting of MGE- and POA-derived interneurons in the deep and superficial migratory stream.
Figures
References
-
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. - PubMed
-
- Anderson SA, Marín O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128:353–363. - PubMed
-
- Borrell V, Yoshimura Y, Callaway EM. Targeted gene delivery to telencephalic inhibitory neurons by directional in utero electroporation. J Neurosci Methods. 2005;143:151–158. - PubMed
-
- Butt SJ, Fuccillo M, Nery S, Noctor S, Kriegstein A, Corbin JG, Fishell G. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
