Saccades during object viewing modulate oscillatory phase in the superior temporal sulcus
- PMID: 22171044
- PMCID: PMC6623894
- DOI: 10.1523/JNEUROSCI.4102-11.2011
Saccades during object viewing modulate oscillatory phase in the superior temporal sulcus
Abstract
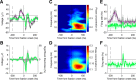
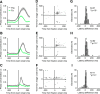
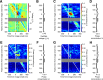
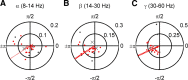
Saccadic eye movements (SEMs) are the primary means of gating visual information in primates and strongly influence visual perception. The active exploration of the visual environment ("active vision") via SEMs produces suppression during saccades and enhancement afterward (i.e., during fixation) in occipital visual areas. In lateral temporal lobe visual areas, the influence, if any, of eye movements is less well understood, despite the necessity of these areas for forming coherent percepts of objects. The upper bank of the superior temporal sulcus (uSTS) is one such area whose sensitivity to SEMs is unknown. We therefore examined how saccades modulate local field potentials (LFPs) in the uSTS of macaque monkeys while they viewed face and nonface object stimuli. LFP phase concentration increased following fixation onset in the alpha (8-14 Hz), beta (14-30 Hz), and gamma (30-60 Hz) bands and was distinct from the image-evoked response. Furthermore, near-coincident onsets of fixation and image presentation--like those occurring in active vision--led to enhanced responses through greater phase concentration in the same frequency bands. Finally, single-unit activity was modulated by the phase of alpha, beta, and gamma oscillations, suggesting that the observed phase-locking influences spike timing in uSTS. Previous research implicates phase concentration in these frequency bands as a correlate of perceptual performance (Womelsdorf et al., 2006; Bosman et al., 2009). Together, these results demonstrate sensitivity to eye movements in an object-processing region of the brain and represent a plausible neural basis for the enhancement of object processing during active vision.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
