Local diversity and fine-scale organization of receptive fields in mouse visual cortex
- PMID: 22171051
- PMCID: PMC3758577
- DOI: 10.1523/JNEUROSCI.2974-11.2011
Local diversity and fine-scale organization of receptive fields in mouse visual cortex
Abstract
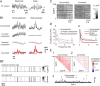
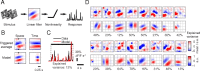
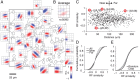
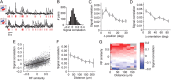
Many thousands of cortical neurons are activated by any single sensory stimulus, but the organization of these populations is poorly understood. For example, are neurons in mouse visual cortex--whose preferred orientations are arranged randomly--organized with respect to other response properties? Using high-speed in vivo two-photon calcium imaging, we characterized the receptive fields of up to 100 excitatory and inhibitory neurons in a 200 μm imaged plane. Inhibitory neurons had nonlinearly summating, complex-like receptive fields and were weakly tuned for orientation. Excitatory neurons had linear, simple receptive fields that can be studied with noise stimuli and system identification methods. We developed a wavelet stimulus that evoked rich population responses and yielded the detailed spatial receptive fields of most excitatory neurons in a plane. Receptive fields and visual responses were locally highly diverse, with nearby neurons having largely dissimilar receptive fields and response time courses. Receptive-field diversity was consistent with a nearly random sampling of orientation, spatial phase, and retinotopic position. Retinotopic positions varied locally on average by approximately half the receptive-field size. Nonetheless, the retinotopic progression across the cortex could be demonstrated at the scale of 100 μm, with a magnification of ≈ 10 μm/°. Receptive-field and response similarity were in register, decreasing by 50% over a distance of 200 μm. Together, the results indicate considerable randomness in local populations of mouse visual cortical neurons, with retinotopy as the principal source of organization at the scale of hundreds of micrometers.
Figures




References
-
- Barlow HB. Possible principles underlying the transformation of sensory images. In: Rosenblith WA, editor. Sensory communication. Cambirdge, MA: MIT; 1961.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
