Ceruloplasmin oxidation, a feature of Parkinson's disease CSF, inhibits ferroxidase activity and promotes cellular iron retention
- PMID: 22171055
- PMCID: PMC6623910
- DOI: 10.1523/JNEUROSCI.3768-11.2011
Ceruloplasmin oxidation, a feature of Parkinson's disease CSF, inhibits ferroxidase activity and promotes cellular iron retention
Abstract
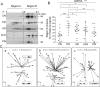
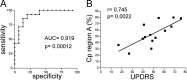
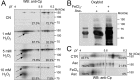
Parkinson's disease is a neurodegenerative disorder characterized by oxidative stress and CNS iron deposition. Ceruloplasmin is an extracellular ferroxidase that regulates cellular iron loading and export, and hence protects tissues from oxidative damage. Using two-dimensional electrophoresis, we investigated ceruloplasmin patterns in the CSF of human Parkinson's disease patients. Parkinson's disease ceruloplasmin profiles proved more acidic than those found in healthy controls and in other human neurological diseases (peripheral neuropathies, amyotrophic lateral sclerosis, and Alzheimer's disease); degrees of acidity correlated with patients' pathological grading. Applying an unsupervised pattern recognition procedure to the two-dimensional electrophoresis images, we identified representative pathological clusters. In vitro oxidation of CSF in two-dimensional electrophoresis generated a ceruloplasmin shift resembling that observed in Parkinson's disease and co-occurred with an increase in protein carbonylation. Likewise, increased protein carbonylation was observed in Parkinson's disease CSF, and the same modification was directly identified in these samples on ceruloplasmin. These results indicate that ceruloplasmin oxidation contributes to pattern modification in Parkinson's disease. From the functional point of view, ceruloplasmin oxidation caused a decrease in ferroxidase activity, which in turn promotes intracellular iron retention in neuronal cell lines as well as in primary neurons, which are more sensitive to iron accumulation. Accordingly, the presence of oxidized ceruloplasmin in Parkinson's disease CSF might be used as a marker for oxidative damage and might provide new insights into the underlying pathological mechanisms.
Figures


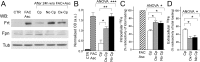
Similar articles
-
Ceruloplasmin functional changes in Parkinson's disease-cerebrospinal fluid.Mol Neurodegener. 2015 Nov 4;10:59. doi: 10.1186/s13024-015-0055-2. Mol Neurodegener. 2015. PMID: 26537957 Free PMC article.
-
Reduced ferroxidase activity in the cerebrospinal fluid from patients with Parkinson's disease.Neurosci Lett. 1999 Apr 23;265(3):155-8. doi: 10.1016/s0304-3940(99)00221-9. Neurosci Lett. 1999. PMID: 10327154
-
Ceruloplasmin regulates iron levels in the CNS and prevents free radical injury.J Neurosci. 2002 Aug 1;22(15):6578-86. doi: 10.1523/JNEUROSCI.22-15-06578.2002. J Neurosci. 2002. PMID: 12151537 Free PMC article.
-
[Ceruloplasmin (Cp) and iron in connection with Parkinson's disease (PD) and Alzheimer's disease (AD)].Laeknabladid. 2012 Oct;98(10):531-7. doi: 10.17992/lbl.2012.10.457. Laeknabladid. 2012. PMID: 23043066 Review. Icelandic.
-
Aceruloplasminemia: an inherited neurodegenerative disease with impairment of iron homeostasis.Ann N Y Acad Sci. 2004 Mar;1012:299-305. doi: 10.1196/annals.1306.024. Ann N Y Acad Sci. 2004. PMID: 15105274 Review.
Cited by
-
In Absence of the Cellular Prion Protein, Alterations in Copper Metabolism and Copper-Dependent Oxidase Activity Affect Iron Distribution.Front Neurosci. 2016 Sep 27;10:437. doi: 10.3389/fnins.2016.00437. eCollection 2016. Front Neurosci. 2016. PMID: 27729845 Free PMC article.
-
[The role of ferroptosis in chronic diseases].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020 May 25;49(1):44-57. doi: 10.3785/j.issn.1008-9292.2020.02.24. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020. PMID: 32621416 Free PMC article. Review. Chinese.
-
A low-molecular-weight ferroxidase is increased in the CSF of sCJD cases: CSF ferroxidase and transferrin as diagnostic biomarkers for sCJD.Antioxid Redox Signal. 2013 Nov 10;19(14):1662-75. doi: 10.1089/ars.2012.5032. Epub 2013 Apr 16. Antioxid Redox Signal. 2013. PMID: 23379482 Free PMC article.
-
Wild-type Cu/Zn-superoxide dismutase is misfolded in cerebrospinal fluid of sporadic amyotrophic lateral sclerosis.Mol Neurodegener. 2019 Nov 19;14(1):42. doi: 10.1186/s13024-019-0341-5. Mol Neurodegener. 2019. PMID: 31744522 Free PMC article.
-
Cerebrospinal fluid ceruloplasmin levels predict cognitive decline and brain atrophy in people with underlying β-amyloid pathology.Neurobiol Dis. 2020 Jun;139:104810. doi: 10.1016/j.nbd.2020.104810. Epub 2020 Feb 19. Neurobiol Dis. 2020. PMID: 32087292 Free PMC article.
References
-
- Baraj B, Cortina JL, Sastre A, Granados M. Copper interference on spectrophotometric determination of iron and their simultaneous determination using bathophenantroline-disulfonic acid disodium salt. Fresenius J Anal Chem. 1998;360:263–265.
-
- Bettegazzi B, Mihailovich M, Di Cesare A, Consonni A, Macco R, Pelizzoni I, Codazzi F, Grohovaz F, Zacchetti D. beta-Secretase activity in rat astrocytes: translational block of BACE1 and modulation of BACE2 expression. Eur J Neurosci. 2011;33:236–243. - PubMed
-
- Bharucha KJ, Friedman JK, Vincent AS, Ross ED. Lower serum ceruloplasmin levels correlate with younger age of onset in Parkinson's disease. J Neurol. 2008;255:1957–1962. - PubMed
-
- Boll MC, Sotelo J, Otero E, Alcaraz-Zubeldia M, Rios C. Reduced ferroxidase activity in the cerebrospinal fluid from patients with Parkinson's disease. Neurosci Lett. 1999;265:155–158. - PubMed
-
- Boll MC, Alcaraz-Zubeldia M, Montes S, Rios C. Free copper, ferroxidase and SOD1 activities, lipid peroxidation and NO(x) content in the CSF. A different marker profile in four neurodegenerative diseases. Neurochem Res. 2008;33:1717–1723. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
