Interactions between "what" and "when" in the auditory system: temporal predictability enhances repetition suppression
- PMID: 22171057
- PMCID: PMC6623902
- DOI: 10.1523/JNEUROSCI.2599-11.2011
Interactions between "what" and "when" in the auditory system: temporal predictability enhances repetition suppression
Abstract
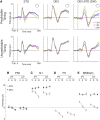
Neural activity in the auditory system decreases with repeated stimulation, matching stimulus probability in multiple timescales. This phenomenon, known as stimulus-specific adaptation, is interpreted as a neural mechanism of regularity encoding aiding auditory object formation. However, despite the overwhelming literature covering recordings from single-cell to scalp auditory-evoked potential (AEP), stimulation timing has received little interest. Here we investigated whether timing predictability enhances the experience-dependent modulation of neural activity associated with stimulus probability encoding. We used human electrophysiological recordings in healthy participants who were exposed to passive listening of sound sequences. Pure tones of different frequencies were delivered in successive trains of a variable number of repetitions, enabling the study of sequential repetition effects in the AEP. In the predictable timing condition, tones were delivered with isochronous interstimulus intervals; in the unpredictable timing condition, interstimulus intervals varied randomly. Our results show that unpredictable stimulus timing abolishes the early part of the repetition positivity, an AEP indexing auditory sensory memory trace formation, while leaving the later part (≈ >200 ms) unaffected. This suggests that timing predictability aids the propagation of repetition effects upstream the auditory pathway, most likely from association auditory cortex (including the planum temporale) toward primary auditory cortex (Heschl's gyrus) and beyond, as judged by the timing of AEP latencies. This outcome calls for attention to stimulation timing in future experiments regarding sensory memory trace formation in AEP measures and stimulus probability encoding in animal models.
Figures


References
-
- Alho K. Cerebral generators of mismatch negativity (MMN) and its magnetic counterpart (MMNm) elicited by sound changes. Ear Hear. 1995;16:38–51. - PubMed
-
- Baess P, Horváth J, Jacobsen T, Schröger E. Selective suppression of self-initiated sounds in an auditory stream: an ERP study. Psychophysiology. 2011;48:1276–1283. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
