Adaptations in glutamate and glycine content within the lumbar spinal cord are associated with the generation of novel gait patterns in rats following neonatal spinal cord transection
- PMID: 22171058
- PMCID: PMC3268368
- DOI: 10.1523/JNEUROSCI.3499-11.2011
Adaptations in glutamate and glycine content within the lumbar spinal cord are associated with the generation of novel gait patterns in rats following neonatal spinal cord transection
Abstract
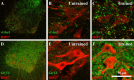
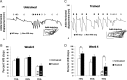
After spinal cord transection, the generation of stepping depends on neurotransmitter systems entirely contained within the local lumbar spinal cord. Glutamate and glycine likely play important roles, but surprisingly little is known about how the content of these two key neurotransmitters changes to achieve weight-bearing stepping after spinal cord injury. We studied the levels of glutamate and glycine in the lumbar spinal cord of spinally transected rats. Rats (n = 48) received spinal cord transection at 5 days of age, and 4 weeks later half were trained to step using a robotic treadmill system and the remaining half were untrained controls. Analyses of glutamate and glycine content via high-performance liquid chromatography showed training significantly raised the levels of both neurotransmitters in the lumbar spinal cord beyond normal. The levels of both neurotransmitters were significantly correlated with the ability to perform independent stepping during training. Glutamate and glycine levels were not significantly different between Untrained and Normal rats or between Trained and Untrained rats. There was a trend for higher expression of VGLUT1 and GLYT2 around motor neurons in Trained versus Untrained rats based on immunohistochemical analyses. Training improved the ability to generate stepping at a range of weight support levels, but normal stepping characteristics were not restored. These findings suggested that the remodeling of the lumbar spinal circuitry in Trained spinally transected rats involved adaptations in the glutamatergic and glycinergic neurotransmitter systems. These adaptations may contribute to the generation of novel gait patterns following complete spinal cord transection.
Figures


Similar articles
-
VGLUT1 and GLYT2 labeling of sacrocaudal motoneurons in the spinal cord injured spastic rat.Exp Neurol. 2007 Mar;204(1):195-204. doi: 10.1016/j.expneurol.2006.10.008. Epub 2006 Nov 28. Exp Neurol. 2007. PMID: 17134699
-
Hindlimb loading determines stepping quantity and quality following spinal cord transection.Brain Res. 2005 Jul 19;1050(1-2):180-9. doi: 10.1016/j.brainres.2005.05.041. Brain Res. 2005. PMID: 15979592
-
Changes in innervation of lumbar motoneurons and organization of premotor network following training of transected adult rats.Exp Neurol. 2018 Jan;299(Pt A):1-14. doi: 10.1016/j.expneurol.2017.09.002. Epub 2017 Sep 14. Exp Neurol. 2018. PMID: 28917641
-
Transplants and neurotrophic factors increase regeneration and recovery of function after spinal cord injury.Prog Brain Res. 2002;137:257-73. doi: 10.1016/s0079-6123(02)37020-1. Prog Brain Res. 2002. PMID: 12440372 Review.
-
Can the mammalian lumbar spinal cord learn a motor task?Med Sci Sports Exerc. 1994 Dec;26(12):1491-7. Med Sci Sports Exerc. 1994. PMID: 7869884 Review.
Cited by
-
Maladaptive spinal plasticity opposes spinal learning and recovery in spinal cord injury.Front Physiol. 2012 Oct 10;3:399. doi: 10.3389/fphys.2012.00399. eCollection 2012. Front Physiol. 2012. PMID: 23087647 Free PMC article.
-
Glycine and N-Acetylcysteine (GlyNAC) Combined with Body Weight Support Treadmill Training Improved Spinal Cord and Skeletal Muscle Structure and Function in Rats with Spinal Cord Injury.Nutrients. 2023 Oct 28;15(21):4578. doi: 10.3390/nu15214578. Nutrients. 2023. PMID: 37960231 Free PMC article.
-
Habilitation Improves Mouse Gait Development Following Neonatal Brain Injury.Prog Rehabil Med. 2022 Dec 1;7:20220061. doi: 10.2490/prm.20220061. eCollection 2022. Prog Rehabil Med. 2022. PMID: 36479304 Free PMC article.
-
Functional Recovery from Neural Stem/Progenitor Cell Transplantation Combined with Treadmill Training in Mice with Chronic Spinal Cord Injury.Sci Rep. 2016 Aug 3;6:30898. doi: 10.1038/srep30898. Sci Rep. 2016. PMID: 27485458 Free PMC article.
-
Robot-Applied Resistance Augments the Effects of Body Weight-Supported Treadmill Training on Stepping and Synaptic Plasticity in a Rodent Model of Spinal Cord Injury.Neurorehabil Neural Repair. 2017 Aug;31(8):746-757. doi: 10.1177/1545968317721016. Epub 2017 Jul 25. Neurorehabil Neural Repair. 2017. PMID: 28741434 Free PMC article.
References
-
- Alvarez FJ, Villalba RM, Zerda R, Schneider SP. Vesicular glutamate transporters in the spinal cord, with special reference to sensory primary afferent synapses. J Comp Neurol. 2004;472:257–280. - PubMed
-
- Cha J, Heng C, Reinkensmeyer DJ, Roy RR, Edgerton VR, de Leon RD. Locomotor ability in spinal rats is dependent on the amount of activity imposed on the hindlimbs during treadmill training. J Neurotrauma. 2007;24:1000–1012. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
