The costs of evolving resistance in heterogeneous parasite environments
- PMID: 22171085
- PMCID: PMC3311890
- DOI: 10.1098/rspb.2011.2259
The costs of evolving resistance in heterogeneous parasite environments
Abstract
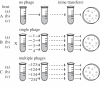
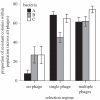
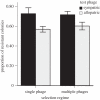
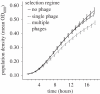
The evolution of host resistance to parasites, shaped by associated fitness costs, is crucial for epidemiology and maintenance of genetic diversity. Selection imposed by multiple parasites could be a particularly strong constraint, as hosts either accumulate costs of multiple specific resistances or evolve a more costly general resistance mechanism. We used experimental evolution to test how parasite heterogeneity influences the evolution of host resistance. We show that bacterial host populations evolved specific resistance to local bacteriophage parasites, regardless of whether they were in single or multiple-phage environments, and that hosts evolving with multiple phages were no more resistant to novel phages than those evolving with single phages. However, hosts from multiple-phage environments paid a higher cost, in terms of population growth in the absence of phage, for their evolved specific resistances than those from single-phage environments. Given that in nature host populations face selection pressures from multiple parasite strains and species, our results suggest that costs may be even more critical in shaping the evolution of resistance than previously thought. Furthermore, our results highlight that a better understanding of resistance costs under combined control strategies could lead to a more 'evolution-resistant' treatment of disease.
Figures
Similar articles
-
The cost of phage resistance in a plant pathogenic bacterium is context-dependent.Evolution. 2015 May;69(5):1321-8. doi: 10.1111/evo.12652. Epub 2015 Apr 27. Evolution. 2015. PMID: 25809535 Free PMC article.
-
The role of parasites in sympatric and allopatric host diversification.Nature. 2002 Dec 5;420(6915):496-9. doi: 10.1038/nature01164. Nature. 2002. PMID: 12466840
-
Genomic and phenotypic signatures of bacteriophage coevolution with the phytopathogen Pseudomonas syringae.Mol Ecol. 2024 May;33(10):e16850. doi: 10.1111/mec.16850. Epub 2023 Feb 5. Mol Ecol. 2024. PMID: 36651263
-
Multifaceted Impacts of Bacteriophages in the Plant Microbiome.Annu Rev Phytopathol. 2018 Aug 25;56:361-380. doi: 10.1146/annurev-phyto-080417-045858. Epub 2018 Jun 29. Annu Rev Phytopathol. 2018. PMID: 29958076 Review.
-
Viral host-adaptation: insights from evolution experiments with phages.Curr Opin Virol. 2013 Oct;3(5):572-7. doi: 10.1016/j.coviro.2013.07.001. Epub 2013 Jul 25. Curr Opin Virol. 2013. PMID: 23890845 Review.
Cited by
-
A single transcription factor facilitates an insect host combating Bacillus thuringiensis infection while maintaining fitness.Nat Commun. 2022 Oct 12;13(1):6024. doi: 10.1038/s41467-022-33706-x. Nat Commun. 2022. PMID: 36224245 Free PMC article.
-
Fitness Trade-Offs in Phage Cocktail-Resistant Salmonella enterica Serovar Enteritidis Results in Increased Antibiotic Susceptibility and Reduced Virulence.Microbiol Spectr. 2022 Oct 26;10(5):e0291422. doi: 10.1128/spectrum.02914-22. Epub 2022 Sep 27. Microbiol Spectr. 2022. PMID: 36165776 Free PMC article.
-
Cross-resistance is modular in bacteria-phage interactions.PLoS Biol. 2018 Oct 3;16(10):e2006057. doi: 10.1371/journal.pbio.2006057. eCollection 2018 Oct. PLoS Biol. 2018. PMID: 30281587 Free PMC article.
-
Exploring the risks of phage application in the environment.Front Microbiol. 2013 Nov 29;4:358. doi: 10.3389/fmicb.2013.00358. eCollection 2013. Front Microbiol. 2013. PMID: 24348468 Free PMC article. Review.
-
Coevolutionary analysis of Pseudomonas syringae-phage interactions to help with rational design of phage treatments.Microb Biotechnol. 2024 Jun;17(6):e14489. doi: 10.1111/1751-7915.14489. Microb Biotechnol. 2024. PMID: 38864499 Free PMC article.
References
-
- Bérénos C., Schmid-Hempel P., Wegner M. K. 2009. Evolution of host resistance and trade-offs between virulence and transmission potential in an obligately killing parasite. J. Evol. Biol. 22, 2049–205610.1111/j.1420-9101.2009.01821.x (doi:10.1111/j.1420-9101.2009.01821.x) - DOI - DOI - PubMed
-
- Boots M., Begon M. 1993. Trade-offs with resistance to a granulosis virus in the Indian meal moth, examined by a laboratory evolution experiment. Funct. Ecol. 7, 528–53410.2307/2390128 (doi:10.2307/2390128) - DOI - DOI
-
- Fuxa J. R., Richter A. R. 1989. Reversion of resistance by Spodoptera frugiperda to nuclear polyhedrosis virus. J. Invertebr. Pathol. 53, 52–5610.1016/0022-2011(89)90073-6 (doi:10.1016/0022-2011(89)90073-6) - DOI - DOI
-
- Koskella B., Lively C. M. 2007. Advice of the rose: experimental coevolution of a trematode parasite and its snail host. Evolution 61, 152–15910.1111/j.1558-5646.2007.00012.x (doi:10.1111/j.1558-5646.2007.00012.x) - DOI - DOI - PubMed
-
- Kraaijeveld A. R., Godfray H. C. J. 1997. Trade-off between parasitoid resistance and larval competitive ability in Drosophila melanogaster. Nature 389, 278–28010.1038/38483 (doi:10.1038/38483) - DOI - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

