Cytotoxic T lymphocytes mediate chronic inflammation of the nasal mucosa of patients with atypical allergic rhinitis
- PMID: 22171246
- PMCID: PMC3234139
- DOI: 10.4297/najms.2011.3378
Cytotoxic T lymphocytes mediate chronic inflammation of the nasal mucosa of patients with atypical allergic rhinitis
Abstract
Background: The prevalence of chronic rhinitis is increasing rapidly; its pathogenesis is to be further understood; immune inflammation is one of the possible causative factors. Antigen specific CD8+ T cells play a critical role in the induction of chronic inflammation.
Aims: This study aimed to investigate the role of antigen specific CD8+ T cells in the pathogenesis of chronic atypical allergic rhinitis.
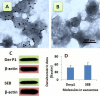
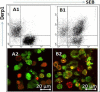
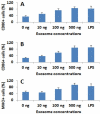
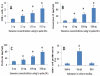
Material and methods: Nasal mucosal epithelial surface scratching samples were obtained from patients with chronic obstruction atypical allergic rhinitis. Exosomes were purified from the scratching samples and examined by immune gold electron microscopy. The effect of exosomes on modulating dendritic cell's properties, the effect of exosome-pulsed dendritic cells on naïve T cell differentiation and the antigen specific CD8+ T cell activation were observed by cell culture models.
Results: Exosomes purified from patients with chronic atypical allergic rhinitis carried microbial products, Staphylococcal enterotoxin B (SEB), and airborne antigen, Derp1. Dendritic cells pulsed by SEB/Derp1-carrying exosomes showed high levels of CD80, CD86 and the major histocompatibility class I (MHCI). Exosome-pulsed dendritic cells could induce the naïve CD3+ T cells to differentiate into CD8+ T cells. Upon the exposure to a specific antigen, the CD8+ T cells released granzyme B and perforin; more than 30% antigen specific CD8+ T cells proliferated.
Conclusions: Antigen specific CD8+ T cells play an important role in the pathogenesis of chronic obstruction atypical allergic rhinitis.
Keywords: Antigen specific response; CD8 T lymphocytes; Epithelium; Rhinitis.
Figures

Similar articles
-
Antigen-specific activities of CD8+ T cells in the nasal mucosa of patients with nasal allergy.Asian Pac J Allergy Immunol. 2012 Jun;30(2):107-13. Asian Pac J Allergy Immunol. 2012. PMID: 22830289
-
Mucosal T-cell phenotypes in persistent atopic and nonatopic rhinitis show an association with mast cells.Allergy. 2004 Feb;59(2):204-12. doi: 10.1046/j.1398-9995.2003.00315.x. Allergy. 2004. PMID: 14763935
-
Increased expression of HLA-DR and CD86 in nasal epithelial cells in allergic rhinitics: antigen presentation to T cells and up-regulation by diesel exhaust particles.Clin Exp Allergy. 2007 Mar;37(3):420-33. doi: 10.1111/j.1365-2222.2007.02672.x. Clin Exp Allergy. 2007. PMID: 17359392 Free PMC article.
-
Nasal sensitization.Allergy. 1997;52(33 Suppl):7-9. doi: 10.1111/j.1398-9995.1997.tb04797.x. Allergy. 1997. PMID: 9188940 Review.
-
Pathophysiology of allergic and nonallergic rhinitis.Proc Am Thorac Soc. 2011 Mar;8(1):106-14. doi: 10.1513/pats.201008-057RN. Proc Am Thorac Soc. 2011. PMID: 21364228 Review.
Cited by
-
Plasma EVs Display Antigen-Presenting Characteristics in Patients With Allergic Rhinitis and Promote Differentiation of Th2 Cells.Front Immunol. 2021 Oct 8;12:710372. doi: 10.3389/fimmu.2021.710372. eCollection 2021. Front Immunol. 2021. PMID: 34691024 Free PMC article.
-
Sinonasal T-cell expression of cytotoxic mediators granzyme B and perforin is reduced in patients with chronic rhinosinusitis.Am J Rhinol Allergy. 2017 Nov 1;31(6):352-356. doi: 10.2500/ajra.2017.31.4474. Am J Rhinol Allergy. 2017. PMID: 29122079 Free PMC article.
-
Effect of immunostimulation with bacterial lysate on the clinical course of allergic rhinitis and the level of γδT, iNKT and cytotoxic T cells in children sensitized to grass pollen allergens: A randomized controlled trial.Front Immunol. 2023 Jan 17;14:1073788. doi: 10.3389/fimmu.2023.1073788. eCollection 2023. Front Immunol. 2023. PMID: 36733480 Free PMC article. Clinical Trial.
-
Functions of Exosomes and Microbial Extracellular Vesicles in Allergy and Contact and Delayed-Type Hypersensitivity.Int Arch Allergy Immunol. 2016;171(1):1-26. doi: 10.1159/000449249. Epub 2016 Nov 8. Int Arch Allergy Immunol. 2016. PMID: 27820941 Free PMC article. Review.
-
The impact of allergen exposure and specific immunotherapy on circulating blood cells in allergic rhinitis.World Allergy Organ J. 2018 Aug 15;11(1):19. doi: 10.1186/s40413-018-0197-0. eCollection 2018. World Allergy Organ J. 2018. PMID: 30128065 Free PMC article. Review.
References
-
- Suh JD, Kennedy DW. Treatment Options for Chronic Rhinosinusitis. Proc Am Thorac Soc. 2011;8:132–140. - PubMed
-
- Timperley D, Schlosser RJ, Harvey RJ. Chronic rhinosinusitis: An education and treatment model. Otolaryngol Head Neck Surg. 2010;143:S3–S8. - PubMed
-
- Tomassen P, Zele TV, Zhang N, Perez-Novo C, Bruaene NV, Gevaert P, Bachert C. Pathophysiology of Chronic Rhinosinusitis. Proc Am Thorac Soc. 2011;8:115–120. - PubMed
-
- Greiner AN, Meltzer EO. Overview of the Treatment of Allergic Rhinitis and Nonallergic Rhinopathy. Proc Am Thorac Soc. 2011;8:121–31. - PubMed
-
- Wang X, Cutting GR. Chronic rhinosinusitis. Adv Otorhinolaryngol. 2011:114–21. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

