Antibody colocalization microarray: a scalable technology for multiplex protein analysis in complex samples
- PMID: 22171321
- PMCID: PMC3322566
- DOI: 10.1074/mcp.M111.011460
Antibody colocalization microarray: a scalable technology for multiplex protein analysis in complex samples
Abstract
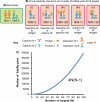
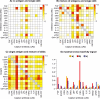
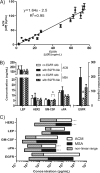
DNA microarrays were rapidly scaled up from 256 to 6.5 million targets, and although antibody microarrays were proposed earlier, sensitive multiplex sandwich assays have only been scaled up to a few tens of targets. Cross-reactivity, arising because detection antibodies are mixed, is a known weakness of multiplex sandwich assays that is mitigated by lengthy optimization. Here, we introduce (1) vulnerability as a metric for assays. The vulnerability of multiplex sandwich assays to cross-reactivity increases quadratically with the number of targets, and together with experimental results, substantiates that scaling up of multiplex sandwich assays is unfeasible. We propose (2) a novel concept for multiplexing without mixing named antibody colocalization microarray (ACM). In ACMs, both capture and detection antibodies are physically colocalized by spotting to the same two-dimensional coordinate. Following spotting of the capture antibodies, the chip is removed from the arrayer, incubated with the sample, placed back onto the arrayer and then spotted with the detection antibodies. ACMs with up to 50 targets were produced, along with a binding curve for each protein. The ACM was validated by comparing it to ELISA and to a small-scale, conventional multiplex sandwich assay (MSA). Using ACMs, proteins in the serum of breast cancer patients and healthy controls were quantified, and six candidate biomarkers identified. Our results indicate that ACMs are sensitive, robust, and scalable.
Figures



References
-
- Moore G. E. (2003) No exponential is forever: but “Forever” can be delayed! Proceedings of the IEEE Solid-State Circuits Conference (ISSCC), pp. 20–23 vol. 21
-
- Ragoussis J., Elvidge G. (2006) Affymetrix GeneChip® system: moving from research to the clinic. Exp. Rev. Mol. Diagnostics 6, 145–152 - PubMed
-
- van de Vijver M. J., He Y. D., van't Veer L. J., Dai H., Hart A. A., Voskuil D. W., Schreiber G. J., Peterse J. L., Roberts C., Marton M. J., Parrish M., Atsma D., Witteveen A., Glas A., Delahaye L., van der Velde T., Bartelink H., Rodenhuis S., Rutgers E. T., Friend S. H., Bernards R. (2002) A gene-expression signature as a predictor of survival in breast cancer. N. Engl. J. Med. 347, 1999–2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

