Mimotopes selected with neutralizing antibodies against multiple subtypes of influenza A
- PMID: 22171803
- PMCID: PMC3320565
- DOI: 10.1186/1743-422X-8-542
Mimotopes selected with neutralizing antibodies against multiple subtypes of influenza A
Abstract
Background: The mimotopes of viruses are considered as the good targets for vaccine design. We prepared mimotopes against multiple subtypes of influenza A and evaluate their immune responses in flu virus challenged Balb/c mice.
Methods: The mimotopes of influenza A including pandemic H1N1, H3N2, H2N2 and H1N1 swine-origin influenza virus were screened by peptide phage display libraries, respectively. These mimotopes were engineered in one protein as multi- epitopes in Escherichia coli (E. coli) and purified. Balb/c mice were immunized using the multi-mimotopes protein and specific antibody responses were analyzed using hemagglutination inhibition (HI) assay and enzyme-linked immunosorbent assay (ELISA). The lung inflammation level was evaluated by hematoxylin and eosin (HE).
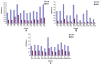
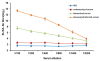
Results: Linear heptopeptide and dodecapeptide mimotopes were obtained for these influenza virus. The recombinant multi-mimotopes protein was a 73 kDa fusion protein. Comparing immunized infected groups with unimmunized infected subsets, significant differences were observed in the body weight loss and survival rate. The antiserum contained higher HI Ab titer against H1N1 virus and the lung inflammation level were significantly decreased in immunized infected groups.
Conclusions: Phage-displayed mimotopes against multiple subtypes of influenza A were accessible to the mouse immune system and triggered a humoral response to above virus.
Figures

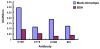


Similar articles
-
Influence of adjuvants on the amount, specificity and functional activity of antibody response to human influenza vaccine in mice.Mol Immunol. 2021 Jul;135:398-407. doi: 10.1016/j.molimm.2021.05.003. Epub 2021 May 20. Mol Immunol. 2021. PMID: 34022515
-
Inactivated H7 Influenza Virus Vaccines Protect Mice despite Inducing Only Low Levels of Neutralizing Antibodies.J Virol. 2017 Sep 27;91(20):e01202-17. doi: 10.1128/JVI.01202-17. Print 2017 Oct 15. J Virol. 2017. PMID: 28768855 Free PMC article.
-
Potential Role of Nonneutralizing IgA Antibodies in Cross-Protective Immunity against Influenza A Viruses of Multiple Hemagglutinin Subtypes.J Virol. 2020 Jun 1;94(12):e00408-20. doi: 10.1128/JVI.00408-20. Print 2020 Jun 1. J Virol. 2020. PMID: 32269119 Free PMC article.
-
Mimotope vaccines: epitope mimics induce anti-cancer antibodies.Immunol Lett. 2007 Oct 31;113(1):1-5. doi: 10.1016/j.imlet.2007.07.008. Epub 2007 Aug 22. Immunol Lett. 2007. PMID: 17825923 Free PMC article. Review.
-
Epitopes and Mimotopes Identification Using Phage Display for Vaccine Development against Infectious Pathogens.Vaccines (Basel). 2023 Jun 29;11(7):1176. doi: 10.3390/vaccines11071176. Vaccines (Basel). 2023. PMID: 37514992 Free PMC article. Review.
Cited by
-
Architectural insight into inovirus-associated vectors (IAVs) and development of IAV-based vaccines inducing humoral and cellular responses: implications in HIV-1 vaccines.Viruses. 2014 Dec 17;6(12):5047-76. doi: 10.3390/v6125047. Viruses. 2014. PMID: 25525909 Free PMC article. Review.
-
ITEM-THREE analysis of a monoclonal anti-malaria antibody reveals its assembled epitope on the pfMSP119 antigen.J Biol Chem. 2020 Oct 30;295(44):14987-14997. doi: 10.1074/jbc.RA120.014802. Epub 2020 Aug 26. J Biol Chem. 2020. PMID: 32848020 Free PMC article.
-
Rapid Response to Pandemic Threats: Immunogenic Epitope Detection of Pandemic Pathogens for Diagnostics and Vaccine Development Using Peptide Microarrays.J Proteome Res. 2020 Nov 6;19(11):4339-4354. doi: 10.1021/acs.jproteome.0c00484. Epub 2020 Sep 21. J Proteome Res. 2020. PMID: 32892628 Free PMC article. Review.
-
The development of inovirus-associated vector vaccines using phage-display technologies.Expert Rev Vaccines. 2019 Sep;18(9):913-920. doi: 10.1080/14760584.2019.1651649. Epub 2019 Sep 8. Expert Rev Vaccines. 2019. PMID: 31373843 Free PMC article. Review.
-
Screening and identification of mimotopes of the major shrimp allergen tropomyosin using one-bead-one-compound peptide libraries.Cell Mol Immunol. 2017 Mar;14(3):308-318. doi: 10.1038/cmi.2015.83. Epub 2015 Sep 14. Cell Mol Immunol. 2017. PMID: 26364917 Free PMC article.
References
-
- Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, Sessions WM, Xu X, Skepner E, Deyde V, Okomo-Adhiambo M, Gubareva L, Barnes J, Smith CB, Emery SL, Hillman MJ, Rivailler P, Smagala J, de Graaf M, Burke DF, Fouchier RA, Pappas C, Alpuche-Aranda CM, López-Gatell H, Olivera H, López I, Myers CA, Faix D, Blair PJ, Yu C, Keene KM, Dotson PD Jr, Boxrud D, Sambol AR, Abid SH, St George K, Bannerman T, Moore AL, Stringer DJ, Blevins P, Demmler-Harrison GJ, Ginsberg M, Kriner P, Waterman S, Smole S, Guevara HF, Belongia EA, Clark PA, Beatrice ST, Donis R, Katz J, Finelli L, Bridges CB, Shaw M, Jernigan DB, Uyeki TM, Smith DJ, Klimov AI, Cox NJ. Antigenic and genetic characteristics of swine-origin 2009 A (H1N1) influenza virus circulating in humans. Science. 2009;325:197–201. doi: 10.1126/science.1176225. - DOI - PMC - PubMed
-
- Sui J, Hwang WC, Perez S, Wei G, Aird D, Chen LM, Santelli E, Stec B, Cadwell G, Ali M, Wan H, Murakami A, Yammanuru A, Han T, Cox NJ, Bankston LA, Donis RO, Liddington RC, Marasco WA. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat Struct Mol Biol. 2009;16:265–73. doi: 10.1038/nsmb.1566. - DOI - PMC - PubMed
-
- Zakay-Rones Z. Human influenza vaccines and assessment of immunogenicity. Expert Rev Vaccines. 2010;17:1423–39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

