Mouse models for studying pain in sickle disease: effects of strain, age, and acuteness
- PMID: 22171826
- PMCID: PMC3779783
- DOI: 10.1111/j.1365-2141.2011.08977.x
Mouse models for studying pain in sickle disease: effects of strain, age, and acuteness
Abstract
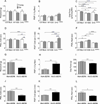
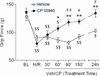
The clinical management of severe pain associated with sickle cell disease (SCD) remains challenging. Development of an optimal therapy would be facilitated by use of murine model(s) with varying degrees of sickling and pain tests that are most sensitive to vaso-occlusion. We found that young (≤3 months old) NY1DD and S+S(Antilles) mice (having modest and moderate sickle phenotype, respectively) exhibited evidence of deep tissue/musculoskeletal pain. Deep tissue pain and cold sensitivity in S+S(Antilles) mice increased significantly with both age and incitement of hypoxia/reoxygenation (H/R). C57/BL6 mice (genetic background strain of NY1DD and S+S(Antilles) ) were hypersensitive to mechanical and heat stimuli, even without the sickle transgene. H/R treatment of HbSS-BERK mice with severe sickle phenotype resulted in significantly decreased withdrawal thresholds and enhanced mechanical, thermal and deep tissue hyperalgesia. Deep hyperalgesia incited by H/R in HbSS-BERK was ameliorated by CP 55940, a cannabinoid receptor agonist. Thus, assessment of deep tissue pain appears to be the most sensitive measure for studying pain mechanisms across mouse models of SCD, and HbSS-BERK mice may be the best model for vaso-occlusive and chronic pain of SCD.
© 2011 Blackwell Publishing Ltd.
Figures


References
-
- Ballas SK. Current issues in sickle cell pain and its management. Hematology Am Soc Hematol Educ Program. 2007:97–105. - PubMed
-
- Belcher JD, Bryant CJ, Nguyen J, Bowlin PR, Kielbik MC, Bischof JC, Hebbel RP, Vercellotti GM. Transgenic sickle mice have vascular inflammation. Blood. 2003;101:3953–3959. - PubMed
-
- Carden DL Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol. 2000;190:255–266. - PubMed
-
- Chaplan SR, Pogrel JW, Yaksh TL. Role of voltage-dependent calcium channel subtypes in experimental tactile allodynia. J Pharmacol Exp Ther. 1994;269:1117–1123. - PubMed
-
- Dampier C, Ely B, Brodecki D, O’Neal P. Characteristics of pain managed at home in children and adolescents with sickle cell disease by using diary self-reports. J Pain. 2002;3:461–470. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

