Nitric oxide influences auxin signaling through S-nitrosylation of the Arabidopsis TRANSPORT INHIBITOR RESPONSE 1 auxin receptor
- PMID: 22171938
- PMCID: PMC3324642
- DOI: 10.1111/j.1365-313X.2011.04885.x
Nitric oxide influences auxin signaling through S-nitrosylation of the Arabidopsis TRANSPORT INHIBITOR RESPONSE 1 auxin receptor
Abstract
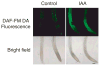
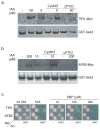
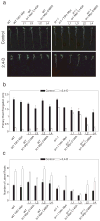
Previous studies have demonstrated that auxin (indole-3-acetic acid) and nitric oxide (NO) are plant growth regulators that coordinate several plant physiological responses determining root architecture. Nonetheless, the way in which these factors interact to affect these growth and developmental processes is not well understood. The Arabidopsis thaliana F-box proteins TRANSPORT INHIBITOR RESPONSE 1/AUXIN SIGNALING F-BOX (TIR1/AFB) are auxin receptors that mediate degradation of AUXIN/INDOLE-3-ACETIC ACID (Aux/IAA) repressors to induce auxin-regulated responses. A broad spectrum of NO-mediated protein modifications are known in eukaryotic cells. Here, we provide evidence that NO donors increase auxin-dependent gene expression while NO depletion blocks Aux/IAA protein degradation. NO also enhances TIR1-Aux/IAA interaction as evidenced by pull-down and two-hybrid assays. In addition, we provide evidence for NO-mediated modulation of auxin signaling through S-nitrosylation of the TIR1 auxin receptor. S-nitrosylation of cysteine is a redox-based post-translational modification that contributes to the complexity of the cellular proteome. We show that TIR1 C140 is a critical residue for TIR1-Aux/IAA interaction and TIR1 function. These results suggest that TIR1 S-nitrosylation enhances TIR1-Aux/IAA interaction, facilitating Aux/IAA degradation and subsequently promoting activation of gene expression. Our findings underline the importance of NO in phytohormone signaling pathways.
© 2011 The Authors. The Plant Journal © 2011 Blackwell Publishing Ltd.
Figures




References
-
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. - PubMed
-
- Correa-Aragunde N, Graziano M, Lamattina L. Nitric oxide plays a central role in determining lateral root development in tomato. Planta. 2004;218:900–905. - PubMed
-
- Correa-Aragunde N, Lanteri ML, Garcia-Mata C, ten Have A, Laxalt AM, Graziano M, Lamattina L. Nitric oxide functions as intermediate in auxin, abscisic acid, and lipid signaling pathways. In: Lamattina L, Polacco JC, editors. Nitric Oxide in Plant Growth, Development and Stress Physiology. Berlin, Germany: Springer-Verlag Berlin Heidelberg; 2007. pp. 113–130.
-
- Delledonne M. NO news is good news for plants. Curr Opin Plant Biol. 2005;8:390–396. - PubMed
-
- Dharmasiri N, Dharmasiri S, Estelle M. The F-box protein TIR1 is an auxin receptor. Nature. 2005a;435:441–445. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

