SOD mimetics: a novel class of androgen receptor inhibitors that suppresses castration-resistant growth of prostate cancer
- PMID: 22172488
- PMCID: PMC3256291
- DOI: 10.1158/1535-7163.MCT-11-0540
SOD mimetics: a novel class of androgen receptor inhibitors that suppresses castration-resistant growth of prostate cancer
Abstract
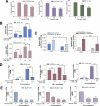
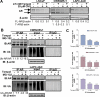
Advanced prostate cancer is the second leading cause of cancer-related deaths among American men. The androgen receptor (AR) is vital for prostate cancer progression, even in the face of castrate levels of serum testosterone following androgen ablation therapy, a mainstay therapy for advanced prostate cancer. Downregulation of superoxide dismutase 2 (SOD2), a major intracellular antioxidant enzyme, occurs progressively during prostate cancer progression to advanced states and is known to promote AR activity in prostate cancer. Therefore, this study investigated the effects of SOD mimetics on AR expression and function in AR-dependent LNCaP, CWR22Rv1, and LAPC-4AD prostate cancer cells. Treatment with Tempol (4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl), a SOD mimetic, not only lowered cellular superoxide levels but also concomitantly attenuated AR transcriptional activity and AR target gene expression in a dose- and time-dependent manner, in the presence and absence of dihydrotestosterone, the major endogenous AR agonist. Inhibition of AR by Tempol was mediated, in large part, by its ability to decrease AR protein via increased degradation, in the absence of any inhibitory effects on other nuclear receptors. Inhibitory effects of Tempol on AR were also reproducible with other SOD mimetics, MnTBAP and MnTMPyP. Importantly, effects of Tempol on AR function were accompanied by significant in vitro and in vivo reduction in castration-resistant prostate cancer (CRPC) survival and growth. Collectively, this study has shown for the first time that SOD mimetics, by virtue of their ability to suppress AR function, may be beneficial in treating the currently incurable CRPC, in which SOD2 expression is highly suppressed.
©2011 AACR.
Figures



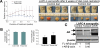
Similar articles
-
Cryptotanshinone suppresses androgen receptor-mediated growth in androgen dependent and castration resistant prostate cancer cells.Cancer Lett. 2012 Mar;316(1):11-22. doi: 10.1016/j.canlet.2011.10.006. Epub 2011 Oct 10. Cancer Lett. 2012. PMID: 22154085 Free PMC article.
-
Growth Inhibition by Testosterone in an Androgen Receptor Splice Variant-Driven Prostate Cancer Model.Prostate. 2016 Dec;76(16):1536-1545. doi: 10.1002/pros.23238. Epub 2016 Jul 30. Prostate. 2016. PMID: 27473672
-
Effects of manganese superoxide dismutase silencing on androgen receptor function and gene regulation: implications for castration-resistant prostate cancer.Clin Cancer Res. 2008 Oct 1;14(19):6073-80. doi: 10.1158/1078-0432.CCR-08-0591. Clin Cancer Res. 2008. PMID: 18829485 Free PMC article.
-
Androgen receptor-dependent and -independent mechanisms driving prostate cancer progression: Opportunities for therapeutic targeting from multiple angles.Oncotarget. 2017 Jan 10;8(2):3724-3745. doi: 10.18632/oncotarget.12554. Oncotarget. 2017. PMID: 27741508 Free PMC article. Review.
-
Bipolar Androgen Therapy Followed by Androgen Receptor Inhibition as Sequential Therapy for Prostate Cancer.Oncologist. 2023 Jun 2;28(6):465-473. doi: 10.1093/oncolo/oyad055. Oncologist. 2023. PMID: 37027449 Free PMC article. Review.
Cited by
-
A Genetic Variation of SOD2 Does Not Determine Duration of Response to Androgen Deprivation Therapy for Prostate Cancer.Prostate. 2016 Oct;76(14):1338-41. doi: 10.1002/pros.23220. Epub 2016 Jun 21. Prostate. 2016. PMID: 27325180 Free PMC article.
-
MiRNAs and tempol therapeutic potential in prostate cancer: a preclinical approach.J Mol Histol. 2025 Jan 13;56(1):69. doi: 10.1007/s10735-024-10341-y. J Mol Histol. 2025. PMID: 39804465
-
The Activity of Superoxide Dismutase, Its Relationship with the Concentration of Zinc and Copper and the Prevalence of rs2070424 Superoxide Dismutase Gene in Women with Polycystic Ovary Syndrome-Preliminary Study.J Clin Med. 2022 May 1;11(9):2548. doi: 10.3390/jcm11092548. J Clin Med. 2022. PMID: 35566673 Free PMC article.
-
Cytoplasmic irradiation results in mitochondrial dysfunction and DRP1-dependent mitochondrial fission.Cancer Res. 2013 Nov 15;73(22):6700-10. doi: 10.1158/0008-5472.CAN-13-1411. Epub 2013 Sep 30. Cancer Res. 2013. PMID: 24080278 Free PMC article.
-
Inflammation-mediated SOD-2 upregulation contributes to epithelial-mesenchymal transition and migration of tumor cells in aflatoxin G1-induced lung adenocarcinoma.Sci Rep. 2017 Aug 11;7(1):7953. doi: 10.1038/s41598-017-08537-2. Sci Rep. 2017. PMID: 28801561 Free PMC article.
References
-
- Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. Jama. 2005;294:238–44. - PubMed
-
- Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23:8253–61. - PubMed
-
- Grossmann ME, Huang H, Tindall DJ. Androgen receptor signaling in androgen-refractory prostate cancer. J Natl Cancer Inst. 2001;93:1687–97. - PubMed
-
- Liao X, Tang S, Thrasher JB, Griebling TL, Li B. Small-interfering RNA-induced androgen receptor silencing leads to apoptotic cell death in prostate cancer. Mol Cancer Ther. 2005;4:505–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

