Neuroendocrine dysfunction in polycystic ovary syndrome
- PMID: 22172593
- PMCID: PMC3453528
- DOI: 10.1016/j.steroids.2011.12.007
Neuroendocrine dysfunction in polycystic ovary syndrome
Abstract
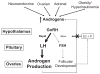
Polycystic ovarian syndrome (PCOS) is a common disorder characterized by ovulatory dysfunction and hyperandrogenemia (HA). Neuroendocrine abnormalities including increased gonadotropin-releasing hormone (GnRH) pulse frequency, increased luteinizing hormone (LH) pulsatility, and relatively decreased follicle stimulating hormone contribute to its pathogenesis. HA reduces inhibition of GnRH pulse frequency by progesterone, causing rapid LH pulse secretion and increasing ovarian androgen production. The origins of persistently rapid GnRH secretion are unknown but appear to evolve during puberty. Obese girls are at risk for HA and develop increased LH pulse frequency with elevated mean LH by late puberty. However, even early pubertal girls with HA have increased LH pulsatility and enhanced daytime LH pulse secretion, indicating the abnormalities may begin early in puberty. Decreasing sensitivity to progesterone may regulate normal maturation of LH secretion, potentially related to normally increasing levels of testosterone during puberty. This change in sensitivity may become exaggerated in girls with HA. Many girls with HA-especially those with hyperinsulinemia-do not exhibit normal LH pulse sensitivity to progesterone inhibition. Thus, HA may adversely affect LH pulse regulation during pubertal maturation leading to persistent HA and the development of PCOS.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures



References
-
- Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223–36. - PubMed
-
- Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–9. - PubMed
-
- Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care. 1999;22:141–6. - PubMed
-
- Franks S. Adult polycystic ovary syndrome begins in childhood. Best Pract Res Clin Endocrinol Metab. 2002;16:263–72. - PubMed
-
- Witchel SF. Puberty and polycystic ovary syndrome. Mol Cell Endocrinol. 2006:254–255. 146–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

