β-catenin signaling controls metastasis in Braf-activated Pten-deficient melanomas
- PMID: 22172720
- PMCID: PMC3241928
- DOI: 10.1016/j.ccr.2011.10.030
β-catenin signaling controls metastasis in Braf-activated Pten-deficient melanomas
Abstract
Malignant melanoma is characterized by frequent metastasis, however, specific changes that regulate this process have not been clearly delineated. Although it is well known that Wnt signaling is frequently dysregulated in melanoma, the functional implications of this observation are unclear. By modulating β-catenin levels in a mouse model of melanoma that is based on melanocyte-specific Pten loss and Braf(V600E) mutation, we demonstrate that β-catenin is a central mediator of melanoma metastasis to the lymph nodes and lungs. In addition to altering metastasis, β-catenin levels control tumor differentiation and regulate both MAPK/Erk and PI3K/Akt signaling. Highly metastatic tumors with β-catenin stabilization are very similar to a subset of human melanomas. Together these findings establish Wnt signaling as a metastasis regulator in melanoma.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
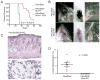

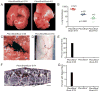

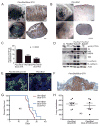


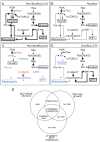
References
-
- Bedrosian I, Faries MB, Guerry D, 4th, Elenitsas R, Schuchter L, Mick R, Spitz FR, Bucky LP, Alavi A, Elder DE, et al. Incidence of sentinel node metastasis in patients with thin primary melanoma (< or = 1mm) with vertical growth phase. Ann Surg Oncol. 2000;7:262–267. - PubMed
-
- Bennett DC, Dexter TJ, Ormerod EJ, Hart IR. Increased experimental metastatic capability of a murine melanoma following induction of differentiation. Cancer Res. 1986;46:3239–3244. - PubMed
-
- Boussadia O, Kutsch S, Hierholzer A, Delmas V, Kemler R. E-cadherin is a survival factor for the lactating mouse mammary gland. Mech Dev. 2002;115:53–62. - PubMed
-
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the β-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

