Oncogene-targeting T cells reject large tumors while oncogene inactivation selects escape variants in mouse models of cancer
- PMID: 22172721
- PMCID: PMC3658305
- DOI: 10.1016/j.ccr.2011.10.019
Oncogene-targeting T cells reject large tumors while oncogene inactivation selects escape variants in mouse models of cancer
Abstract
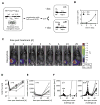
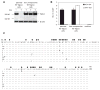
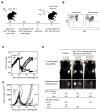
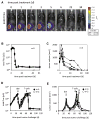
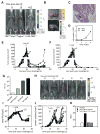
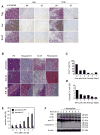
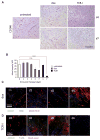
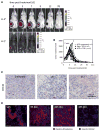
The genetic instability of cancer cells frequently causes drug resistance. We established mouse cancer models, which allowed targeting of an oncogene by drug-mediated inactivation or monospecific CD8(+) effector T (T(E)) cells. Drug treatment of genetically unstable large tumors was effective but selected resistant clones in the long term. In contrast, T(E) cells completely rejected large tumors (≥500 mm(3)), if the target antigen was cancer-driving and expressed in sufficient amounts. Although drug-mediated oncogene inactivation selectively killed the cancer cells and left the tumor vasculature intact, which likely facilitated survival and growth of resistant clones, T(E) cell treatment led to blood vessel destruction and probably "bystander" elimination of escape variants, which did not require antigen cross-presentation by stromal cells.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
References
-
- Blankenstein T. The role of tumor stroma in the interaction between tumor and immune system. Curr Opin Immunol. 2005;17:180–186. - PubMed
-
- Blohm U, Potthoff D, van der Kogel AJ, Pircher H. Solid tumors “melt” from the inside after successful CD8 T cell attack. Eur J Immunol. 2006;36:468–477. - PubMed
-
- Briesemeister D, Sommermeyer D, Loddenkemper C, Loew R, Uckert W, Blankenstein T, Kammertoens T. Tumor rejection by local interferon-γ induction in established tumors is associated with blood vessel destruction and necrosis. Int J Cancer. 2010;128:371–378. - PubMed
-
- Buschow C, Charo J, Anders K, Loddenkemper C, Jukica A, Alsamah W, Perez C, Willimsky G, Blankenstein T. In vivo imaging of an inducible oncogenic tumor antigen visualizes tumor progression and predicts CTL tolerance. J Immunol. 2010;184:2930–2938. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

