Mechanism of protein biosynthesis in mammalian mitochondria
- PMID: 22172991
- PMCID: PMC3314146
- DOI: 10.1016/j.bbagrm.2011.11.009
Mechanism of protein biosynthesis in mammalian mitochondria
Abstract
Protein synthesis in mammalian mitochondria produces 13 proteins that are essential subunits of the oxidative phosphorylation complexes. This review provides a detailed outline of each phase of mitochondrial translation including initiation, elongation, termination, and ribosome recycling. The roles of essential proteins involved in each phase are described. All of the products of mitochondrial protein synthesis in mammals are inserted into the inner membrane. Several proteins that may help bind ribosomes to the membrane during translation are described, although much remains to be learned about this process. Mutations in mitochondrial or nuclear genes encoding components of the translation system often lead to severe deficiencies in oxidative phosphorylation, and a summary of these mutations is provided. This article is part of a Special Issue entitled: Mitochondrial Gene Expression.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures



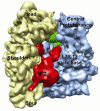

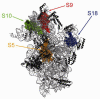




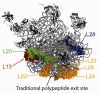
References
-
- Temperley R, Richter R, Dennerlein S, Lightowlers RN, Chrzanowska- Lightowlers ZM. Hungry codons promote frameshifting in human mitochondrial ribosomes. Science. 2010;327:301. - PubMed
-
- Taanman J-W. The mitochondrial genome: structure, transcription, translation and replication. Biochim. Biophys. Acta. 1999;1410:103–123. - PubMed
-
- O’Brien TW. The general occurrence of 55 S ribosomes in mammalian liver mitochondria. J. Biol. Chem. 1971;246:3409–3417. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

