Secretory phospholipase A2-mediated depletion of phosphatidylglycerol in early acute respiratory distress syndrome
- PMID: 22173044
- PMCID: PMC3307942
- DOI: 10.1097/MAJ.0b013e318239c96c
Secretory phospholipase A2-mediated depletion of phosphatidylglycerol in early acute respiratory distress syndrome
Abstract
Introduction: Secretory phospholipases A2 (sPLA2) hydrolyze phospholipids in cell membranes and extracellular structures such as pulmonary surfactant. This study tests the hypothesis that sPLA2 are elevated in human lungs during acute respiratory distress syndrome (ARDS) and that sPLA2 levels are associated with surfactant injury by hydrolysis of surfactant phospholipids.
Methods: Bronchoalveolar lavage (BAL) fluid was obtained from 18 patients with early ARDS (<72 hours) and compared with samples from 10 healthy volunteers. Secreted phospholipase A2 levels were measured (enzyme activity and enzyme immunoassay) in conjunction with ARDS subjects' surfactant abnormalities including surfactant phospholipid composition, large and small aggregates distribution and surface tension function.
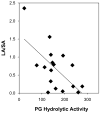
Results: BAL sPLA2 enzyme activity was markedly elevated in ARDS samples relative to healthy subjects when measured by ex vivo hydrolysis of both phosphatidylglycerol (PG) and phosphatidylcholine (PC). Enzyme immunoassay identified increased PLA2G2A protein in the ARDS BAL fluid, which was strongly correlated with the sPLA2 enzyme activity against PG. Of particular interest, the authors demonstrated an average depletion of 69% of the PG in the ARDS sample large aggregates relative to the normal controls. Furthermore, the sPLA2 enzyme activity against PG and PC ex vivo correlated with the BAL recovery of in vivo PG and PC, respectively, and also correlated with the altered distribution of the large and small surfactant aggregates.
Conclusions: These results support the hypothesis that sPLA2-mediated hydrolysis of surfactant phospholipid, especially PG by PLA2G2A, contributes to surfactant injury during early ARDS.
Figures



References
-
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. - PubMed
-
- Zuo YY, Veldhuizen RA, Neumann AW, et al. Current perspectives in pulmonary surfactant--inhibition, enhancement and evaluation. Biochim Biophys Acta. 2008;1778:1947–1977. - PubMed
-
- Kim DK, Fukuda T, Thompson BT, et al. Bronchoalveolar lavage fluid phospholipase A2 activities are increased in human adult respiratory distress syndrome. American Journal of Physiology - Lung Cellular & Molecular Physiology. 1995;269:L109–L118. - PubMed
-
- Holm BA, Keicher L, Liu MY, et al. Inhibition of pulmonary surfactant function by phospholipases. J Appl Physiol. 1991;71:317–321. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

