Spinal projections of the A5, A6 (locus coeruleus), and A7 noradrenergic cell groups in rats
- PMID: 22173709
- PMCID: PMC3508755
- DOI: 10.1002/cne.23024
Spinal projections of the A5, A6 (locus coeruleus), and A7 noradrenergic cell groups in rats
Abstract
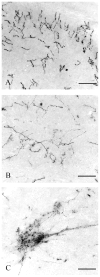
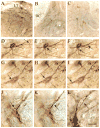
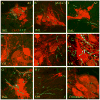
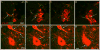
The pontine noradrenergic cell groups, A5, A6 (locus coeruleus), and A7, provide the only noradrenergic innervation of the spinal cord, but the individual contribution of each of these populations to the regional innervation of the spinal cord remains controversial. We used an adeno-associated viral (AAV) vector encoding green fluorescent protein under an artificial dopamine beta-hydroxylase (PRSx8) promoter to trace the spinal projections from the A5, A6, and A7 groups. Projections from all three groups travel through the spinal cord in both the lateral and ventral funiculi and in the dorsal surface of the dorsal horn, but A6 axons take predominantly the dorsal and ventral routes, whereas A5 axons take mainly a lateral and A7 axons a ventral route. The A6 group provides the densest innervation at all levels, and includes all parts of the spinal gray matter, but it is particularly dense in the dorsal horn. The A7 group provides the next most dense innervation, again including all parts of the spinal cord, but is it denser in the ventral horn. The A5 group supplies only sparse innervation to the dorsal and ventral horns and to the cervical and lumbosacral levels, but provides the densest innervation to the thoracic intermediolateral cell column, and in particular to the sympathetic preganglionic neurons. Thus, the pontine noradrenergic cell groups project in a roughly topographic and complementary fashion onto the spinal cord. The pattern of spinal projections observed suggests that the locus coeruleus might have the greatest effect on somatosensory transmission, the A7 group on motor function, and the A5 group on sympathetic function.
Copyright © 2011 Wiley Periodicals, Inc.
Figures
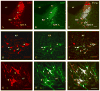
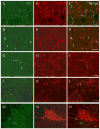
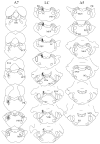
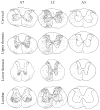

References
-
- Anderson CR, McLachlan EM, Srb-Christie O. Distribution of sympathetic preganglionic neurons and monoaminergic nerve terminals in the spinal cord of the rat. J Comp Neurol. 1989;283(2):269–84. - PubMed
-
- Balcita-Pedicino JJ, Rinaman L. Noradrenergic axon terminals contact gastric preautonomic neurons in the paraventricular nucleus of the hypothalamus in rats. J Comp Neurol. 2007;501(4):608–18. - PubMed
-
- Barber RP, Phelps PE, Houser CR, Crawford GD, Salvaterra PM, Vaughn JE. The morphology and distribution of neurons containing choline acetyltransferase in the adult rat spinal cord: an immunocytochemical study. J Comp Neurol. 1984;229(3):329–46. - PubMed
-
- Batton RR, III, Jayaraman A, Ruggiero D, Carpenter MB. Fastigial efferent projections in the monkey: an autoradiographic study. J Comp Neurol. 1977;174(2):281–305. - PubMed
-
- Broekman ML, Comer LA, Hyman BT, Sena-Esteves M. Adeno-associated virus vectors serotyped with AAV8 capsid are more efficient than AAV-1 or -2 serotypes for widespread gene delivery to the neonatal mouse brain. Neuroscience. 2006;138(2):501–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

