Effects of Olig2-overexpressing neural stem cells and myelin basic protein-activated T cells on recovery from spinal cord injury
- PMID: 22173726
- PMCID: PMC3337015
- DOI: 10.1007/s13311-011-0090-9
Effects of Olig2-overexpressing neural stem cells and myelin basic protein-activated T cells on recovery from spinal cord injury
Abstract
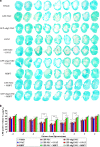
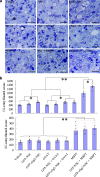
Neural stem cell (NSC) transplantation is a major focus of current research for treatment of spinal cord injury (SCI). However, it is very important to promote the survival and differentiation of NSCs into myelinating oligodendrocytes (OLs). In this study, myelin basic protein-activated T (MBP-T) cells were passively immunized to improve the SCI microenvironment. Olig2-overexpressing NSCs were infected with a lentivirus carrying the enhanced green fluorescent protein (GFP) reporter gene to generate Olig2-GFP-NSCs that were transplanted into the injured site to differentiate into OLs. Transferred MBP-T cells infiltrated the injured spinal cord, produced neurotrophic factors, and induced the differentiation of resident microglia and/or infiltrating blood monocytes into an "alternatively activated" anti-inflammatory macrophage phenotype by producing interleukin-13. As a result, the survival of transplanted NSCs increased fivefold in MBP-T cell-transferred rats compared with that of the vehicle-treated control. In addition, the differentiation of MBP-positive OLs increased 12-fold in Olig2-GFP-NSC-transplanted rats compared with that of GFP-NSC-transplanted controls. In the MBP-T cell and Olig2-GFP-NSC combined group, the number of OL-remyelinated axons significantly increased compared with those of all other groups. However, a significant decrease in spinal cord lesion volume and an increase in spared myelin and behavioral recovery were observed in Olig2-NSC- and NSC-transplanted MBP-T cell groups. Collectively, these results suggest that MBP-T cell adoptive immunotherapy combined with NSC transplantation has a synergistic effect on histological and behavioral improvement after traumatic SCI. Although Olig2 overexpression enhances OL differentiation and myelination, the effect on functional recovery may be surpassed by MBP-T cells.
Figures







Similar articles
-
Transplantation of human neural stem cells transduced with Olig2 transcription factor improves locomotor recovery and enhances myelination in the white matter of rat spinal cord following contusive injury.BMC Neurosci. 2009 Sep 22;10:117. doi: 10.1186/1471-2202-10-117. BMC Neurosci. 2009. PMID: 19772605 Free PMC article.
-
Differential and cooperative actions of Olig1 and Olig2 transcription factors on immature proliferating cells after contusive spinal cord injury.Glia. 2011 Jul;59(7):1094-106. doi: 10.1002/glia.21182. Epub 2011 May 2. Glia. 2011. PMID: 21538562
-
Cyclosporin A increases recovery after spinal cord injury but does not improve myelination by oligodendrocyte progenitor cell transplantation.BMC Neurosci. 2010 Oct 12;11:127. doi: 10.1186/1471-2202-11-127. BMC Neurosci. 2010. PMID: 20937147 Free PMC article.
-
Advances in genetically modified neural stem cell therapy for central nervous system injury and neurological diseases.Stem Cell Res Ther. 2024 Dec 18;15(1):482. doi: 10.1186/s13287-024-04089-1. Stem Cell Res Ther. 2024. PMID: 39696712 Free PMC article. Review.
-
Oligodendrocyte fate after spinal cord injury.Neurotherapeutics. 2011 Apr;8(2):262-73. doi: 10.1007/s13311-011-0033-5. Neurotherapeutics. 2011. PMID: 21404073 Free PMC article. Review.
Cited by
-
Hematogenous Macrophages: A New Therapeutic Target for Spinal Cord Injury.Front Cell Dev Biol. 2021 Nov 24;9:767888. doi: 10.3389/fcell.2021.767888. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34901013 Free PMC article. Review.
-
Effects of neural stem cell transplantation on the motor function of rats with contusion spinal cord injuries: a meta-analysis.Neural Regen Res. 2020 Apr;15(4):748-758. doi: 10.4103/1673-5374.266915. Neural Regen Res. 2020. PMID: 31638100 Free PMC article.
-
Sonic hedgehog promotes generation and maintenance of human forebrain Olig2 progenitors.Front Cell Neurosci. 2013 Dec 13;7:254. doi: 10.3389/fncel.2013.00254. eCollection 2013. Front Cell Neurosci. 2013. PMID: 24379757 Free PMC article.
-
Transcriptome profile of rat genes in injured spinal cord at different stages by RNA-sequencing.BMC Genomics. 2017 Feb 15;18(1):173. doi: 10.1186/s12864-017-3532-x. BMC Genomics. 2017. PMID: 28201982 Free PMC article.
-
Engineering Stem Cells for Biomedical Applications.Adv Healthc Mater. 2016 Jan 7;5(1):10-55. doi: 10.1002/adhm.201400842. Epub 2015 Mar 13. Adv Healthc Mater. 2016. PMID: 25772134 Free PMC article. Review.
References
-
- Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Morshead CM, Fehlings MG. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci. 2006;26:3377–3389. doi: 10.1523/JNEUROSCI.4184-05.2006. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

