Responses to second-line tyrosine kinase inhibitors are durable: an intention-to-treat analysis in chronic myeloid leukemia patients
- PMID: 22174159
- PMCID: PMC5162552
- DOI: 10.1182/blood-2011-10-383000
Responses to second-line tyrosine kinase inhibitors are durable: an intention-to-treat analysis in chronic myeloid leukemia patients
Abstract
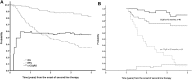
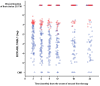
Second-generation tyrosine kinase inhibitors (2G-TKIs) are effective at inducing complete cytogenetic responses (CCyRs) in approximately half of chronic myeloid leukemia patients treated while still in the chronic phase and after failing imatinib. It is less clear whether these responses are durable. In the present study, we report the clinical outcome of 119 patients who received a 2G-TKI as second-line treatment while still in the chronic phase. In an intention-to-treat analysis, the 4-year probabilities of overall and event-free survival were 81.9% and 35.3%, respectively. Sixty-two patients discontinued the initial 2G-TKI because of resistance or intolerance. To further explore the durability of cytogenetic responses, irrespective of the need for a third-line TKI, we used the concept of "current CCyR-survival" (c-CCyRS). The c-CCyRS at 4 years was 54.4%. After introduction of a 2G-TKI, 77 patients had a 3-month BCR-ABL1/ABL1 transcript ratio of ≤ 10% and had significantly superior overall survival (91.3% vs 72.1%, P = .02), event-free survival (49.3% vs 13.0%, P < .001), and c-CCyRS (67.2% vs 11.2%, P = .0001) compared with the 33 patients with ratios > 10%. The 3-month molecular response was the only independent predictor for overall survival. Using an intention-to-treat analysis, we have shown that the responses to second-line therapies are durable. Patients destined to fare poorly can be identified early during therapy.
Figures


References
-
- Talpaz M, Shah NP, Kantarjian H, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354(24):2531–2541. - PubMed
-
- Kantarjian H, Giles F, Wunderle L, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med. 2006;354(24):2542–2551. - PubMed
-
- Shah NP, Kim DW, Kantarjian H, et al. Potent, transient inhibition of BCR-ABL with dasatinib 100 mg daily achieves rapid and durable cytogenetic responses and high transformation-free survival rates in chronic phase chronic myeloid leukemia patients with resistance, suboptimal response or intolerance to imatinib. Haematologica. 2010;95(2):232–240. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

