MST1 mutations in autosomal recessive primary immunodeficiency characterized by defective naive T-cell survival
- PMID: 22174160
- PMCID: PMC3824282
- DOI: 10.1182/blood-2011-09-378364
MST1 mutations in autosomal recessive primary immunodeficiency characterized by defective naive T-cell survival
Abstract
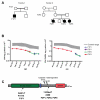
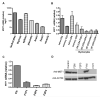
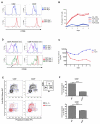
The molecular mechanisms that underlie T-cell quiescence are poorly understood. In the present study, we report a primary immunodeficiency phenotype associated with MST1 deficiency and primarily characterized by a progressive loss of naive T cells. The in vivo consequences include recurrent bacterial and viral infections and autoimmune manifestations. MST1-deficient T cells poorly expressed the transcription factor FOXO1, the IL-7 receptor, and BCL2. Conversely, FAS expression and the FAS-mediating apoptotic pathway were up-regulated. These abnormalities suggest that increased cell death of naive and proliferating T cells is the main mechanism underlying this novel immunodeficiency. Our results characterize a new mechanism in primary T-cell immunodeficiencies and highlight a role of the MST1/FOXO1 pathway in controlling the death of human naive T cells.
Figures


Comment in
-
A loss of naivete.Blood. 2012 Apr 12;119(15):3371-2. doi: 10.1182/blood-2012-02-410399. Blood. 2012. PMID: 22500047
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

