Regulation of cell wall biogenesis in Saccharomyces cerevisiae: the cell wall integrity signaling pathway
- PMID: 22174182
- PMCID: PMC3241422
- DOI: 10.1534/genetics.111.128264
Regulation of cell wall biogenesis in Saccharomyces cerevisiae: the cell wall integrity signaling pathway
Abstract
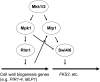
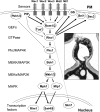
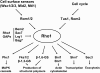
The yeast cell wall is a strong, but elastic, structure that is essential not only for the maintenance of cell shape and integrity, but also for progression through the cell cycle. During growth and morphogenesis, and in response to environmental challenges, the cell wall is remodeled in a highly regulated and polarized manner, a process that is principally under the control of the cell wall integrity (CWI) signaling pathway. This pathway transmits wall stress signals from the cell surface to the Rho1 GTPase, which mobilizes a physiologic response through a variety of effectors. Activation of CWI signaling regulates the production of various carbohydrate polymers of the cell wall, as well as their polarized delivery to the site of cell wall remodeling. This review article centers on CWI signaling in Saccharomyces cerevisiae through the cell cycle and in response to cell wall stress. The interface of this signaling pathway with other pathways that contribute to the maintenance of cell wall integrity is also discussed.
Figures




References
-
- Abe J., Kusuhara M., Ulevitch R. J., Berk B. C., Lee J. D., 1996. Big mitogen-activated protein kinase 1 (BMK1) is a redox-sensitive kinase. J. Biol. Chem. 271: 16586–16590 - PubMed
-
- Aguilar-Uscanga B., François J. M., 2003. A study of the yeast cell wall composition and structure in response to growth conditions and mode of cultivation. Lett. Appl. Microbiol. 37: 268–274 - PubMed
-
- Alberts A. S., 2001. Identification of a carboxyl-terminal diaphanous-related formin homology protein autoregulatory domain. J. Biol. Chem. 276: 2824–2830 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

