Myosin II motor activity in the lateral amygdala is required for fear memory consolidation
- PMID: 22174310
- PMCID: PMC3246591
- DOI: 10.1101/lm.024042.111
Myosin II motor activity in the lateral amygdala is required for fear memory consolidation
Abstract
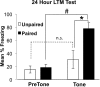
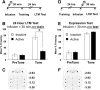
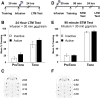
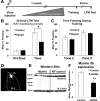
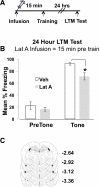
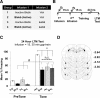
Learning induces dynamic changes to the actin cytoskeleton that are required to support memory formation. However, the molecular mechanisms that mediate filamentous actin (F-actin) dynamics during learning and memory are poorly understood. Myosin II motors are highly expressed in actin-rich growth structures including dendritic spines, and we have recently shown that these molecular machines mobilize F-actin in response to synaptic stimulation and learning in the hippocampus. In this study, we report that Myosin II motors in the rat lateral amygdala (LA) are essential for fear memory formation. Pretraining infusions of the Myosin II inhibitor, blebbistatin (blebb), disrupted long term memory, while short term memory was unaffected. Interestingly, both post-training and pretesting infusions had no effect on memory formation, indicating that Myosin II motors operate during or shortly after learning to promote memory consolidation. These data support the idea that Myosin II motor-force generation is a general mechanism that supports memory consolidation in the mammalian CNS.
Figures
References
-
- Allingham JS, Smith R, Rayment I 2005. The structural basis of blebbistatin inhibition and specificity for myosin II. Nat Struct Mol Biol 12: 378–379 - PubMed
-
- Blair HT, Schafe GE, Bauer EP, Rodrigues SM, LeDoux JE 2001. Synaptic plasticity in the lateral amygdala: A cellular hypothesis of fear conditioning. Learn Mem 8: 229–242 - PubMed
-
- Brown ME, Bridgman PC 2003. Retrograde flow rate is increased in growth cones from myosin IIB knockout mice. J Cell Sci 116: 1087–1094 - PubMed
-
- Brown ME, Bridgman PC 2004. Myosin function in nervous and sensory systems. J Neurobiol 58: 118–130 - PubMed
-
- Dillon C, Goda Y 2005. The actin cytoskeleton: Integrating form and function at the synapse. Annu Rev Neurosci 28: 25–55 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
