Monocytic CCR2(+) myeloid-derived suppressor cells promote immune escape by limiting activated CD8 T-cell infiltration into the tumor microenvironment
- PMID: 22174368
- PMCID: PMC3288305
- DOI: 10.1158/0008-5472.CAN-11-1792
Monocytic CCR2(+) myeloid-derived suppressor cells promote immune escape by limiting activated CD8 T-cell infiltration into the tumor microenvironment
Abstract
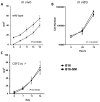
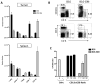
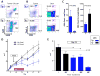
Myeloid-derived suppressor cells (MDSC) are a heterogeneous population of cells that accumulate during tumor formation, facilitate immune escape, and enable tumor progression. MDSCs are important contributors to the development of an immunosuppressive tumor microenvironment that blocks the action of cytotoxic antitumor T effector cells. Heterogeneity in these cells poses a significant barrier to studying the in vivo contributions of individual MDSC subtypes. Herein, we show that granulocyte-macrophage colony stimulating factor, a cytokine critical for the numeric and functional development of MDSC populations, promotes expansion of a monocyte-derived MDSC population characterized by expression of CD11b and the chemokine receptor CCR2. Using a toxin-mediated ablation strategy to target CCR2-expressing cells, we show that these monocytic MDSCs regulate entry of activated CD8 T cells into the tumor site, thereby limiting the efficacy of immunotherapy. Our results argue that therapeutic targeting of monocytic MDSCs would enhance outcomes in immunotherapy.
Conflict of interest statement
The authors have no conflicting interests.
Figures

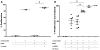

References
-
- Peranzoni E, Zilio S, Marigo I, et al. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 2010;22:238–244. - PubMed
-
- Small E, Demkow T, Gerritsen WR, et al. A phase III trial of GVAX immunotherapy for prostate cancer in combination with docetaxel vs. docetaxel plus prednisone in symptomatic, castration-resistant prostate cancer (CRPC) ASCO GU; 2009. p. Abs 7.
Publication types
MeSH terms
Substances
Grants and funding
- R25 CA020449/CA/NCI NIH HHS/United States
- GM07739/GM/NIGMS NIH HHS/United States
- RC2 CA148468/CA/NCI NIH HHS/United States
- P01 CA033049/CA/NCI NIH HHS/United States
- KO8 AI071998/AI/NIAID NIH HHS/United States
- P01 CA059350/CA/NCI NIH HHS/United States
- T32 GM007739/GM/NIGMS NIH HHS/United States
- P01 CA33049/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- R01 CA56821/CA/NCI NIH HHS/United States
- R01 CA056821/CA/NCI NIH HHS/United States
- R37 AI039031/AI/NIAID NIH HHS/United States
- K08 AI071998/AI/NIAID NIH HHS/United States
- P01 CA59350/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

