Helminth protection against autoimmune diabetes in nonobese diabetic mice is independent of a type 2 immune shift and requires TGF-β
- PMID: 22174447
- PMCID: PMC3253252
- DOI: 10.4049/jimmunol.1100335
Helminth protection against autoimmune diabetes in nonobese diabetic mice is independent of a type 2 immune shift and requires TGF-β
Abstract
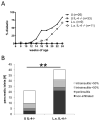
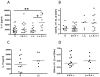
Leading hypotheses to explain helminth-mediated protection against autoimmunity postulate that type 2 or regulatory immune responses induced by helminth infections in the host limit pathogenic Th1-driven autoimmune responses. We tested these hypotheses by investigating whether infection with the filarial nematode Litomosoides sigmodontis prevents diabetes onset in IL-4-deficient NOD mice and whether depletion or absence of regulatory T cells, IL-10, or TGF-β alters helminth-mediated protection. In contrast to IL-4-competent NOD mice, IL-4-deficient NOD mice failed to develop a type 2 shift in either cytokine or Ab production during L. sigmodontis infection. Despite the absence of a type 2 immune shift, infection of IL-4-deficient NOD mice with L. sigmodontis prevented diabetes onset in all mice studied. Infections in immunocompetent and IL-4-deficient NOD mice were accompanied by increases in CD4(+)CD25(+)Foxp3(+) regulatory T cell frequencies and numbers, respectively, and helminth infection increased the proliferation of CD4(+)Foxp3(+) cells. However, depletion of CD25(+) cells in NOD mice or Foxp3(+) T cells from splenocytes transferred into NOD.scid mice did not decrease helminth-mediated protection against diabetes onset. Continuous depletion of the anti-inflammatory cytokine TGF-β, but not blockade of IL-10 signaling, prevented the beneficial effect of helminth infection on diabetes. Changes in Th17 responses did not seem to play an important role in helminth-mediated protection against autoimmunity, because helminth infection was not associated with a decreased Th17 immune response. This study demonstrates that L. sigmodontis-mediated protection against diabetes in NOD mice is not dependent on the induction of a type 2 immune shift but does require TGF-β.
Figures

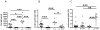


References
-
- Cooke A, Tonks P, Jones FM, O’Shea H, Hutchings P, Fulford AJ, Dunne DW. Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol. 1999;21:169–176. - PubMed
-
- Correale J, Farez M. Association between parasite infection and immune responses in multiple sclerosis. Ann Neurol. 2007;61:97–108. - PubMed
-
- Elliott DE, Setiawan T, Metwali A, Blum A, Urban JF, Jr, Weinstock JV. Heligmosomoides polygyrus inhibits established colitis in IL-10-deficient mice. Eur J Immunol. 2004;34:2690–2698. - PubMed
-
- Fleming JO, Cook TD. Multiple sclerosis and the hygiene hypothesis. Neurology. 2006;67:2085–2086. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

