In Vivo Toxicity of Silver Nanoparticles and Silver Ions in Zebrafish (Danio rerio)
- PMID: 22174711
- PMCID: PMC3235887
- DOI: 10.1155/2012/293784
In Vivo Toxicity of Silver Nanoparticles and Silver Ions in Zebrafish (Danio rerio)
Abstract
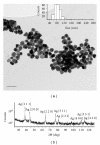
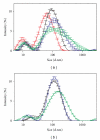
The influence of water chemistry on characterised polyvinyl pyrrolidone- (PVP-) coated silver nanoparticles (81 nm) was investigated. NaCl solution series of 100-800 mg L(-1) lead to initial and temporal increase in nanoparticles size, but agglomeration was limited. pH variation (5-8) had only minor influence on the hydrodynamic particle size. Acute toxicity of nanosivler to zebrafish (Danio rerio) was investigated in a 48-hour static renewal study and compared with the toxicity of silver ions (AgNO(3)). The nanosilver and silver ion 48-hour median lethal concentration (LC(50)) values were 84 μg L(-1) and 25 μg L(-1), respectively. To investigate exposure-related stress, the fish behaviour was observed visually after 0, 3, 6, 12, 24, 27, 30, and 48 hours of both nanosilver and ionic silver treatments. These observations revealed increased rate of operculum movement and surface respiration after nanosilver exposure, suggesting respiratory toxicity. The present study demonstrates that silver nanoparticles are lethal to zebrafish.
Figures

Similar articles
-
Critical influence of chloride ions on silver ion-mediated acute toxicity of silver nanoparticles to zebrafish embryos.Nanotoxicology. 2015 Feb;9(1):81-91. doi: 10.3109/17435390.2014.893379. Epub 2014 Mar 14. Nanotoxicology. 2015. PMID: 24625062
-
Comparison of nanosilver and ionic silver toxicity in Daphnia magna and Pimephales promelas.Environ Toxicol Chem. 2012 Nov;31(11):2557-63. doi: 10.1002/etc.1978. Epub 2012 Sep 20. Environ Toxicol Chem. 2012. PMID: 22887018
-
Molecular aspect of silver nanoparticles regulated embryonic development in Zebrafish (Danio rerio) by Oct-4 expression.Chemosphere. 2018 Sep;206:560-567. doi: 10.1016/j.chemosphere.2018.05.018. Epub 2018 May 4. Chemosphere. 2018. PMID: 29778081
-
The effect of silver nanoparticles and silver ions on zebrafish embryos (Danio rerio).Neuro Endocrinol Lett. 2018 Oct;39(4):299-304. Neuro Endocrinol Lett. 2018. PMID: 30531708
-
Assessment of nanosilver toxicity during zebrafish (Danio rerio) development.Chemosphere. 2013 Jun;92(1):59-66. doi: 10.1016/j.chemosphere.2013.02.060. Epub 2013 Mar 30. Chemosphere. 2013. PMID: 23548591
Cited by
-
Extracellular probiotic lipase capped silver nanoparticles as highly efficient broad spectrum antimicrobial agents.RSC Adv. 2018 Sep 6;8(55):31358-31365. doi: 10.1039/c8ra05999c. eCollection 2018 Sep 5. RSC Adv. 2018. PMID: 35548221 Free PMC article.
-
Green Synthesis of Silver Nanoparticles Using the Leaf Extract of the Medicinal Plant, Uvaria narum and Its Antibacterial, Antiangiogenic, Anticancer and Catalytic Properties.Antibiotics (Basel). 2023 Mar 13;12(3):564. doi: 10.3390/antibiotics12030564. Antibiotics (Basel). 2023. PMID: 36978431 Free PMC article.
-
Hydroethanolic extract from Endopleura uchi (Huber) Cuatrecasas and its marker bergenin: Toxicological and pharmacokinetic studies in silico and in vivo on zebrafish.Toxicol Rep. 2020 Jan 27;7:217-232. doi: 10.1016/j.toxrep.2020.01.011. eCollection 2020. Toxicol Rep. 2020. PMID: 32042599 Free PMC article.
-
Nano silver and nano zinc-oxide in surface waters - exposure estimation for Europe at high spatial and temporal resolution.Environ Pollut. 2015 Jan;196:341-9. doi: 10.1016/j.envpol.2014.10.022. Environ Pollut. 2015. PMID: 25463731 Free PMC article.
-
Silver nanoparticle toxicity in the embryonic zebrafish is governed by particle dispersion and ionic environment.Nanotechnology. 2013 Mar 22;24(11):115101. doi: 10.1088/0957-4484/24/11/115101. Epub 2013 Feb 28. Nanotechnology. 2013. PMID: 23449170 Free PMC article.
References
-
- Renner H. Ullmann's Encylopedia of Industrial Chemistry. New York, NY, USA: John Wiley & Sons; 2009. Silver, silver compounds, and silver alloys.
-
- Purcell TW, Peters JJ. Sources of silver in the environment. Environmental Toxicology and Chemistry. 1998;17(4):539–546.
-
- Vandevelde T, Renner H, Schlamp G. Ullmann's Encylopedia of Industrial Chemistry. chapter 10. New York, NY, USA: John Wiley & Sons; 2009. Silver, silver compounds, and silver alloys.
-
- Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. Journal of Colloid and Interface Science. 2004;275(1):177–182. - PubMed
-
- Benn TM, Westerhoff P. Nanoparticle silver released into water from commercially available sock fabrics. Environmental Science and Technology. 2008;42(11):4133–4139. - PubMed
LinkOut - more resources
Full Text Sources

