Intensification of antiretroviral therapy with a CCR5 antagonist in patients with chronic HIV-1 infection: effect on T cells latently infected
- PMID: 22174752
- PMCID: PMC3234247
- DOI: 10.1371/journal.pone.0027864
Intensification of antiretroviral therapy with a CCR5 antagonist in patients with chronic HIV-1 infection: effect on T cells latently infected
Abstract
Objective: The primary objective was to assess the effect of MVC intensification on latently infected CD4(+) T cells in chronically HIV-1-infected patients receiving antiretroviral therapy.
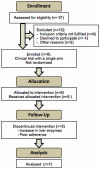
Methods: We performed an open-label pilot phase II clinical trial involving chronically HIV-1-infected patients receiving stable antiretroviral therapy whose regimen was intensified with 48 weeks of maraviroc therapy. We analyzed the latent reservoir, the residual viremia and episomal 2LTR DNA to examine the relationship between these measures and the HIV-1 latent reservoir, immune activation, lymphocyte subsets (including effector and central memory T cells), and markers associated with bacterial translocation.
Results: Overall a non significant reduction in the size of the latent reservoir was found (p = 0.068). A mean reduction of 1.82 IUPM was observed in 4 patients with detectable latent reservoir at baseline after 48 weeks of intensification. No effect on plasma residual viremia was observed. Unexpectedly, all the patients had detectable 2LTR DNA circles at week 24, while none of them showed those circles at the end of the study. No changes were detected in CD4(+) or CD8(+) counts, although a significant decrease was found in the proportion of HLA-DR(+)/CD38(+) CD4(+) and CD8(+) T-cells. LPS and sCD14 levels increased.
Conclusions: Intensification with MVC was associated with a trend to a decrease in the size of the latent HIV-1 reservoir in memory T cells. No impact on residual viremia was detected. Additional studies with larger samples are needed to confirm the results.
Trial registration: ClinicalTrials.gov NCT00795444.
Conflict of interest statement
Figures

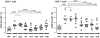

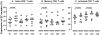
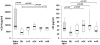
References
-
- Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–5. - PubMed
-
- Anderson JA, Archin NM, Ince W, Parker D, Wiegand A, et al. Clonal sequences recovered from plasma from patients with residual HIV-1 viremia and on intensified antiretroviral therapy are identical to replicating viral RNAs recovered from circulating resting CD4+ T cells. J Virol. 2011;85:5220–3. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

