Characterization of pellicle inhibition in Gluconacetobacter xylinus 53582 by a small molecule, pellicin, identified by a chemical genetics screen
- PMID: 22174763
- PMCID: PMC3235090
- DOI: 10.1371/journal.pone.0028015
Characterization of pellicle inhibition in Gluconacetobacter xylinus 53582 by a small molecule, pellicin, identified by a chemical genetics screen
Abstract
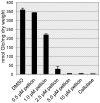
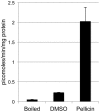
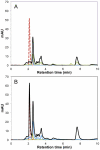
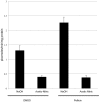
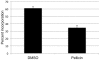
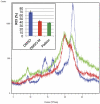
Pellicin ([2E]-3-phenyl-1-[2,3,4,5-tetrahydro-1,6-benzodioxocin-8-yl]prop-2-en-1-one) was identified in a chemical genetics screen of 10,000 small molecules for its ability to completely abolish pellicle production in Gluconacetobacter xylinus. Cells grown in the presence of pellicin grew 1.5 times faster than untreated cells. Interestingly, growth in pellicin also caused G. xylinus cells to elongate. Measurement of cellulose synthesis in vitro showed that cellulose synthase activity was not directly inhibited by pellicin. Rather, when cellulose synthase activity was measured in cells that were pre-treated with the compound, the rate of cellulose synthesis increased eight-fold over that observed for untreated cells. This phenomenon was also apparent in the rapid production of cellulose when cells grown in the presence of pellicin were washed and transferred to media lacking the inhibitor. The rate at which cellulose was produced could not be accounted for by growth of the organism. Pellicin was not detected when intracellular contents were analyzed. Furthermore, it was found that pellicin exerts its effect extracellularly by interfering with the crystallization of pre-cellulosic tactoidal aggregates. This interference of the crystallization process resulted in enhanced production of cellulose II as evidenced by the ratio of acid insoluble to acid soluble product in in vitro assays and confirmed in vivo by scanning electron microscopy and powder X-ray diffraction. The relative crystallinity index, RCI, of pellicle produced by untreated G. xylinus cultures was 70% while pellicin-grown cultures had RCI of 38%. Mercerized pellicle of untreated cells had RCI of 42%, which further confirms the mechanism of action of pellicin as an inhibitor of the cellulose I crystallization process. Pellicin is a useful tool for the study of cellulose biosynthesis in G. xylinus.
Conflict of interest statement
Figures





References
-
- Delmer D, Haigler C. The regulation of metabolic flux to cellulose, a major sink for carbon in plants. Metab Eng. 2002;4:22–28. - PubMed
-
- Fu GZ, Chan AW, Minns DE. Life cycle assessment of bio-ethanol derived from cellulose. Int J LCA. 2003;8:137–141.
-
- Shah J, Brown RM., Jr Towards electronic paper displays made from microbial cellulose. Appl Microbiol Biotechnol. 2005;66:352–355. - PubMed
-
- Czaja W, Krystynowicz A, Bielecki S, Brown R., Jr Microbial cellulose – the natural power to heal wounds. Biomaterials. 2006;27:145–151. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

