Role of collagens and perlecan in microvascular stability: exploring the mechanism of capillary vessel damage by snake venom metalloproteinases
- PMID: 22174764
- PMCID: PMC3234262
- DOI: 10.1371/journal.pone.0028017
Role of collagens and perlecan in microvascular stability: exploring the mechanism of capillary vessel damage by snake venom metalloproteinases
Abstract
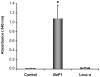
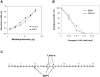
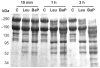
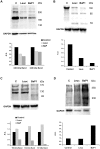
Hemorrhage is a clinically important manifestation of viperid snakebite envenomings, and is induced by snake venom metalloproteinases (SVMPs). Hemorrhagic and non-hemorrhagic SVMPs hydrolyze some basement membrane (BM) and associated extracellular matrix (ECM) proteins. Nevertheless, only hemorrhagic SVMPs are able to disrupt microvessels; the mechanisms behind this functional difference remain largely unknown. We compared the proteolytic activity of the hemorrhagic P-I SVMP BaP1, from the venom of Bothrops asper, and the non-hemorrhagic P-I SVMP leucurolysin-a (leuc-a), from the venom of Bothrops leucurus, on several substrates in vitro and in vivo, focusing on BM proteins. When incubated with Matrigel, a soluble extract of BM, both enzymes hydrolyzed laminin, nidogen and perlecan, albeit BaP1 did it at a faster rate. Type IV collagen was readily digested by BaP1 while leuc-a only induced a slight hydrolysis. Degradation of BM proteins in vivo was studied in mouse gastrocnemius muscle. Western blot analysis of muscle tissue homogenates showed a similar degradation of laminin chains by both enzymes, whereas nidogen was cleaved to a higher extent by BaP1, and perlecan and type IV collagen were readily digested by BaP1 but not by leuc-a. Immunohistochemistry of muscle tissue samples showed a decrease in the immunostaining of type IV collagen after injection of BaP1, but not by leuc-a. Proteomic analysis by LC/MS/MS of exudates collected from injected muscle revealed higher amounts of perlecan, and types VI and XV collagens, in exudates from BaP1-injected tissue. The differences in the hemorrhagic activity of these SVMPs could be explained by their variable ability to degrade key BM and associated ECM substrates in vivo, particularly perlecan and several non-fibrillar collagens, which play a mechanical stabilizing role in microvessel structure. These results underscore the key role played by these ECM components in the mechanical stability of microvessels.
Conflict of interest statement
Figures

Similar articles
-
Insights into the Mechanisms Involved in Strong Hemorrhage and Dermonecrosis Induced by Atroxlysin-Ia, a PI-Class Snake Venom Metalloproteinase.Toxins (Basel). 2017 Aug 2;9(8):239. doi: 10.3390/toxins9080239. Toxins (Basel). 2017. PMID: 28767072 Free PMC article.
-
Key events in microvascular damage induced by snake venom hemorrhagic metalloproteinases.J Proteomics. 2011 Aug 24;74(9):1781-94. doi: 10.1016/j.jprot.2011.03.026. Epub 2011 Apr 6. J Proteomics. 2011. PMID: 21447411 Review.
-
Novel insights into capillary vessel basement membrane damage by snake venom hemorrhagic metalloproteinases: a biochemical and immunohistochemical study.Arch Biochem Biophys. 2006 Nov 15;455(2):144-53. doi: 10.1016/j.abb.2006.09.018. Epub 2006 Oct 6. Arch Biochem Biophys. 2006. PMID: 17055999
-
Local tissue damage induced by BaP1, a metalloproteinase isolated from Bothrops asper (Terciopelo) snake venom.Exp Mol Pathol. 1995 Dec;63(3):186-99. doi: 10.1006/exmp.1995.1042. Exp Mol Pathol. 1995. PMID: 9062552
-
Hemorrhage Caused by Snake Venom Metalloproteinases: A Journey of Discovery and Understanding.Toxins (Basel). 2016 Mar 26;8(4):93. doi: 10.3390/toxins8040093. Toxins (Basel). 2016. PMID: 27023608 Free PMC article. Review.
Cited by
-
P-I metalloproteinases and L-amino acid oxidases from Bothrops species inhibit angiogenesis.J Venom Anim Toxins Incl Trop Dis. 2021 Aug 18;27:e20200180. doi: 10.1590/1678-9199-JVATITD-2020-0180. eCollection 2021. J Venom Anim Toxins Incl Trop Dis. 2021. PMID: 34471403 Free PMC article.
-
Rattlesnake Crotalus molossus nigrescens venom induces oxidative stress on human erythrocytes.J Venom Anim Toxins Incl Trop Dis. 2017 Apr 21;23:24. doi: 10.1186/s40409-017-0114-y. eCollection 2017. J Venom Anim Toxins Incl Trop Dis. 2017. PMID: 28439287 Free PMC article.
-
Proteomic Analysis of Human Blister Fluids Following Envenomation by Three Snake Species in India: Differential Markers for Venom Mechanisms of Action.Toxins (Basel). 2019 Apr 30;11(5):246. doi: 10.3390/toxins11050246. Toxins (Basel). 2019. PMID: 31052189 Free PMC article.
-
Using organ-on-a-chip technology to study haemorrhagic activities of snake venoms on endothelial tubules.Sci Rep. 2024 Jun 4;14(1):11157. doi: 10.1038/s41598-024-60282-5. Sci Rep. 2024. PMID: 38834598 Free PMC article.
-
Insights into the Mechanisms Involved in Strong Hemorrhage and Dermonecrosis Induced by Atroxlysin-Ia, a PI-Class Snake Venom Metalloproteinase.Toxins (Basel). 2017 Aug 2;9(8):239. doi: 10.3390/toxins9080239. Toxins (Basel). 2017. PMID: 28767072 Free PMC article.
References
-
- Fox JW, Serrano SMT. Structural considerations of the snake venom metalloproteinases, key members of the M12 reprolysin family of metalloproteinases. Toxicon. 2005;45:969–985. - PubMed
-
- Calvete JJ, Sanz L, Angulo Y, Lomonte B, Gutiérrez JM. Venoms, venomics, antivenomics. FEBS Lett. 2009;583:1736–1743. - PubMed
-
- Gutiérrez JM, Rucavado A, Escalante T. Snake venom metalloproteinases. Biological roles and participation in the pathophysiology of envenomation. In: Mackessy SP, editor. Handbook of Venoms and Toxins of Reptiles. Boca Raton: CRC Press; 2010. pp. 114–128.
-
- Gutiérrez JM, Rucavado A. Snake venom metalloproteinases: Their role in the pathogenesis of local tissue damage. Biochimie. 2000;82:841–850. - PubMed
-
- Kamiguti AS. Platelets as targets of snake venom metalloproteinases. Toxicon. 2005;45:1041–1049. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

