Functional and transcriptional induction of aquaporin-1 gene by hypoxia; analysis of promoter and role of Hif-1α
- PMID: 22174795
- PMCID: PMC3233559
- DOI: 10.1371/journal.pone.0028385
Functional and transcriptional induction of aquaporin-1 gene by hypoxia; analysis of promoter and role of Hif-1α
Abstract
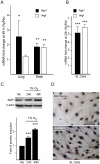
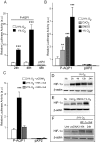
Aquaporin-1 (AQP1) is a water channel that is highly expressed in tissues with rapid O(2) transport. It has been reported that this protein contributes to gas permeation (CO(2), NO and O(2)) through the plasma membrane. We show that hypoxia increases Aqp1 mRNA and protein levels in tissues, namely mouse brain and lung, and in cultured cells, the 9L glioma cell line. Stopped-flow light-scattering experiments confirmed an increase in the water permeability of 9L cells exposed to hypoxia, supporting the view that hypoxic Aqp1 up-regulation has a functional role. To investigate the molecular mechanisms underlying this regulatory process, transcriptional regulation was studied by transient transfections of mouse endothelial cells with a 1297 bp 5' proximal Aqp1 promoter-luciferase construct. Incubation in hypoxia produced a dose- and time-dependent induction of luciferase activity that was also obtained after treatments with hypoxia mimetics (DMOG and CoCl(2)) and by overexpressing stabilized mutated forms of HIF-1α. Single mutations or full deletions of the three putative HIF binding domains present in the Aqp1 promoter partially reduced its responsiveness to hypoxia, and transfection with Hif-1α siRNA decreased the in vitro hypoxia induction of Aqp1 mRNA and protein levels. Our results indicate that HIF-1α participates in the hypoxic induction of AQP1. However, we also demonstrate that the activation of Aqp1 promoter by hypoxia is complex and multifactorial and suggest that besides HIF-1α other transcription factors might contribute to this regulatory process. These data provide a conceptual framework to support future research on the involvement of AQP1 in a range of pathophysiological conditions, including edema, tumor growth, and respiratory diseases.
Conflict of interest statement
Figures




References
-
- Nakhoul NL, Davis BA, Romero MF, Boron WF. Effect of expressing the water channel aquaporin-1 on the CO2 permeability of Xenopus oocytes. Am J Physiol. 1998;274:C543–C548. - PubMed
-
- Herrera M, Hong NJ, Garvin JL. Aquaporin-1 transports NO across cell membranes. Hypertension. 2006;48:157–64. - PubMed
-
- Echevarria M, Muñoz-Cabello AM, Sanchez-Silva R, Toledo-Aral JJ, Lopez-Barneo J. Development of cytosolic hypoxia and HIF stabilization are facilitated by aquaporin 1 expression. J Biol Chem. 2007;282:30207–30215. - PubMed

