Infection of differentiated porcine airway epithelial cells by influenza virus: differential susceptibility to infection by porcine and avian viruses
- PMID: 22174804
- PMCID: PMC3235120
- DOI: 10.1371/journal.pone.0028429
Infection of differentiated porcine airway epithelial cells by influenza virus: differential susceptibility to infection by porcine and avian viruses
Abstract
Background: Swine are important hosts for influenza A viruses playing a crucial role in the epidemiology and interspecies transmission of these viruses. Respiratory epithelial cells are the primary target cells for influenza viruses.
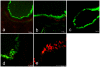
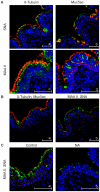
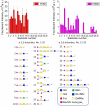
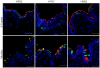
Methodology/principal findings: To analyze the infection of porcine airway epithelial cells by influenza viruses, we established precision-cut lung slices as a culture system for differentiated respiratory epithelial cells. Both ciliated and mucus-producing cells were found to be susceptible to infection by swine influenza A virus (H3N2 subtype) with high titers of infectious virus released into the supernatant already one day after infection. By comparison, growth of two avian influenza viruses (subtypes H9N2 and H7N7) was delayed by about 24 h. The two avian viruses differed both in the spectrum of susceptible cells and in the efficiency of replication. As the H9N2 virus grew to titers that were only tenfold lower than that of a porcine H3N2 virus this avian virus is an interesting candidate for interspecies transmission. Lectin staining indicated the presence of both α-2,3- and α-2,6-linked sialic acids on airway epithelial cells. However, their distribution did not correlate with pattern of virus infection indicating that staining by plant lectins is not a reliable indicator for the presence of cellular receptors for influenza viruses.
Conclusions/significance: Differentiated respiratory epithelial cells significantly differ in their susceptibility to infection by avian influenza viruses. We expect that the newly described precision-cut lung slices from the swine lung are an interesting culture system to analyze the infection of differentiated respiratory epithelial cells by different pathogens (viral, bacterial and parasitic ones) of swine.
Conflict of interest statement
Figures



References
-
- Scholtissek C, Bürger H, Kistner O, Shortridge KF. The nucleoprotein as a possible major factor in determining host specificity of influenza H3N2 viruses. Virology. 1985;147:287–294. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

