BAT3 guides misfolded glycoproteins out of the endoplasmic reticulum
- PMID: 22174835
- PMCID: PMC3234288
- DOI: 10.1371/journal.pone.0028542
BAT3 guides misfolded glycoproteins out of the endoplasmic reticulum
Abstract
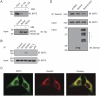
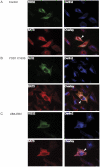
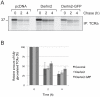
Secretory and membrane proteins that fail to acquire their native conformation within the lumen of the Endoplasmic Reticulum (ER) are usually targeted for ubiquitin-dependent degradation by the proteasome. How partially folded polypeptides are kept from aggregation once ejected from the ER into the cytosol is not known. We show that BAT3, a cytosolic chaperone, is recruited to the site of dislocation through its interaction with Derlin2. Furthermore, we observe cytoplasmic BAT3 in a complex with a polypeptide that originates in the ER as a glycoprotein, an interaction that depends on the cytosolic disposition of both, visualized even in the absence of proteasomal inhibition. Cells depleted of BAT3 fail to degrade an established dislocation substrate. We thus implicate a cytosolic chaperone as an active participant in the dislocation of ER glycoproteins.
Conflict of interest statement
Figures


References
-
- Bagola K, Mehnert M, Jarosch E, Sommer T. Protein dislocation from the ER. Biochim Biophys Acta. 2011;1808:925–936. - PubMed
-
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. - PubMed
-
- Rapoport TA. Protein translocation across the eukaryotic endoplasmic reticulum and bacterial plasma membranes. Nature. 2007;450:663–669. - PubMed
-
- Wiertz EJ, Jones TR, Sun L, Bogyo M, Geuze HJ, et al. The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell. 1996;84:769–779. - PubMed
-
- Wiertz EJ, Tortorella D, Bogyo M, Yu J, Mothes W, et al. Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature. 1996;384:432–438. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

