Sex effects in mouse prion disease incubation time
- PMID: 22174884
- PMCID: PMC3236759
- DOI: 10.1371/journal.pone.0028741
Sex effects in mouse prion disease incubation time
Abstract
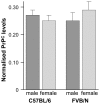
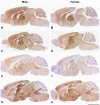
Prion disease incubation time in mice is determined by many factors including PrP expression level, Prnp alleles, genetic background, prion strain and route of inoculation. Sex differences have been described in age of onset for vCJD and in disease duration for both vCJD and sporadic CJD and have also been shown in experimental models. The sex effects reported for mouse incubation times are often contradictory and detail only one strain of mice or prions, resulting in broad generalisations and a confusing picture. To clarify the effect of sex on prion disease incubation time in mice we have compared male and female transmission data from twelve different inbred lines of mice inoculated with at least two prion strains, representing both mouse-adapted scrapie and BSE. Our data show that sex can have a highly significant difference on incubation time. However, this is limited to particular mouse and prion strain combinations. No sex differences were seen in endogenous PrP(C) levels nor in the neuropathological markers of prion disease: PrP(Sc) distribution, spongiosis, neuronal loss and gliosis. These data suggest that when comparing incubation times between experimental groups, such as testing the effects of modifier genes or therapeutics, single sex groups should be used.
Conflict of interest statement
Figures



References
-
- Collinge J. Prion diseases of humans and animals: their causes and molecular basis. Annu Rev Neurosci. 2001;24:519–550. - PubMed
-
- Moore RC, Hope J, McBride PA, McConnell I, Selfridge J, et al. Mice with gene targetted prion protein alterations show that Prnp, Sinc and Prni are congruent. Nature Genet. 1998;18:118–125. - PubMed
-
- Westaway D, Goodman PA, Mirenda CA, McKinley MP, Carlson GA, et al. Distinct prion proteins in short and long scrapie incubation period mice. Cell. 1987;51:651–662. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

