An off-target nucleostemin RNAi inhibits growth in human glioblastoma-derived cancer stem cells
- PMID: 22174890
- PMCID: PMC3236221
- DOI: 10.1371/journal.pone.0028753
An off-target nucleostemin RNAi inhibits growth in human glioblastoma-derived cancer stem cells
Abstract
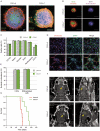
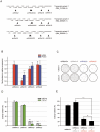
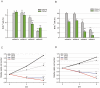
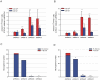
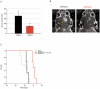
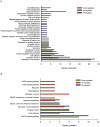
Glioblastomas (GBM) may contain a variable proportion of active cancer stem cells (CSCs) capable of self-renewal, of aggregating into CD133(+) neurospheres, and to develop intracranial tumors that phenocopy the original ones. We hypothesized that nucleostemin may contribute to cancer stem cell biology as these cells share characteristics with normal stem cells. Here we report that nucleostemin is expressed in GBM-CSCs isolated from patient samples, and that its expression, conversely to what it has been described for ordinary stem cells, does not disappear when cells are differentiated. The significance of nucleostemin expression in CSCs was addressed by targeting the corresponding mRNA using lentivirally transduced short hairpin RNA (shRNA). In doing so, we found an off-target nucleostemin RNAi (shRNA22) that abolishes proliferation and induces apoptosis in GBM-CSCs. Furthermore, in the presence of shRNA22, GBM-CSCs failed to form neurospheres in vitro or grow on soft agar. When these cells are xenotransplanted into the brains of nude rats, tumor development is significantly delayed. Attempts were made to identify the primary target/s of shRNA22, suggesting a transcription factor involved in one of the MAP-kinases signaling-pathways or multiple targets. The use of this shRNA may contribute to develop new therapeutic approaches for this incurable type of brain tumor.
Conflict of interest statement
Figures
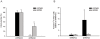
References
-
- Maher EA, Furnari FB, Bachoo RM, Rowitch DH, Louis DN, et al. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15:1311–1333. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, et al. Cancer stem cells–perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. - PubMed
-
- Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. - PubMed

