Combined boyden-flow cytometry assay improves quantification and provides phenotypification of leukocyte chemotaxis
- PMID: 22174892
- PMCID: PMC3235166
- DOI: 10.1371/journal.pone.0028771
Combined boyden-flow cytometry assay improves quantification and provides phenotypification of leukocyte chemotaxis
Abstract
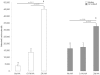
Chemotaxis has been studied by classical methods that measure chemotactic and random motility responses in vitro, but these methods do not evaluate the total number and phenotype of migrating leukocytes simultaneously. Our objective was to develop and validate a novel assay, combined Boyden-flow cytometry chemotaxis assay (CBFCA), for simultaneous quantification and phenotypification of migrating leukocytes. CBFCA exhibited several important advantages in comparison to the classic Boyden chemotaxis assay (CBCA): 1) improved precision (intra-assay coefficients of variation (CVs): CBFCA-4.7 and 4.8% vs. CBCA-30.1 and 17.3%; inter-observer CVs: CBFCA-3.6% vs. CBCA 30.1%); 2) increased recovery of cells, which increased assay to provide increased sensitivity; 3) high specificity for determining the phenotype of migrating/attracted leukocytes; and 4) reduced performance time (CBFCA 120 min vs. CBCA 265 min). Other advantages of CBFCA are: 5) robustness, 6) linearity, 7) eliminated requirement for albumin and, importantly, 8) enabled recovery of migrating leukocytes for subsequent studies. This latter feature is of great benefit in the study of migrating leukocyte subsets. We conclude that the CBFCA is a novel and improved technique for experiments focused on understanding leukocyte trafficking during the inflammatory response.
Conflict of interest statement
Figures



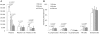
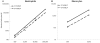
References
-
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. - PubMed
-
- Constantin G, Laudanna C. Leukocyte chemotaxis: from lysosomes to motility. Nat Immunol. 2010;11:463–464. - PubMed
-
- Lauffenburger D, Rothman C, Zigmond SH. Measurement of leukocyte motility and chemotaxis parameters with a linear under-agarose migration assay. J Immunol. 1983;131:940–947. - PubMed
-
- Cutler JE, Munoz JJ. A simple in vitro method for studies on chemotaxis. Proc Soc Exp Biol Med. 1974;147:471–474. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

