Macrophage LRP1 suppresses neo-intima formation during vascular remodeling by modulating the TGF-β signaling pathway
- PMID: 22174911
- PMCID: PMC3235159
- DOI: 10.1371/journal.pone.0028846
Macrophage LRP1 suppresses neo-intima formation during vascular remodeling by modulating the TGF-β signaling pathway
Abstract
Background: Vascular remodeling in response to alterations in blood flow has been shown to modulate the formation of neo-intima. This process results from a proliferative response of vascular smooth muscle cells and is influenced by macrophages, which potentiate the development of the intima. The LDL receptor-related protein 1 (LRP1) is a large endocytic and signaling receptor that recognizes a number of ligands including apoE-containing lipoproteins, proteases and protease-inhibitor complexes. Macrophage LRP1 is known to influence the development of atherosclerosis, but its role in vascular remodeling has not been investigated.
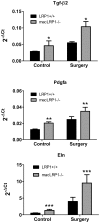
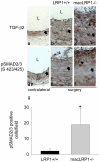
Methodology/principal findings: To define the contribution of macrophage LRP1 to vascular remodeling, we generated macrophage specific LRP1-deficient mice (macLRP1-/-) on an LDL receptor (LDLr) knock-out background. Using a carotid ligation model, we detected a 2-fold increase in neointimal thickening and a 2-fold increase in the intima/media ratio in macLRP1-/- mice. Quantitative RT-PCR arrays of the remodeled vessel wall identified increases in mRNA levels of the TGF-β2 gene as well as the Pdgfa gene in macLRP1-/- mice which could account for the alterations in vascular remodeling. Immunohistochemistry analysis revealed increased activation of the TGF-β signaling pathway in macLRP1-/- mice. Further, we observed that LRP1 binds TGF-β2 and macrophages lacking LRP1 accumulate twice as much TGF-β2 in conditioned media. Finally, TNF-α modulation of the TGF-β2 gene in macrophages is attenuated when LRP1 is expressed. Together, the data reveal that LRP1 modulates both the expression and protein levels of TGF-β2 in macrophages.
Conclusions/significance: Our data demonstrate that macrophage LRP1 protects the vasculature by limiting remodeling events associated with flow. This appears to occur by the ability of macrophage LRP1 to reduce TGF-β2 protein levels and to attenuate expression of the TGF-β2 gene resulting in suppression of the TGF-β signaling pathway.
Conflict of interest statement
Figures
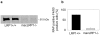




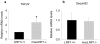

Similar articles
-
Macrophage LRP1 Promotes Diet-Induced Hepatic Inflammation and Metabolic Dysfunction by Modulating Wnt Signaling.Mediators Inflamm. 2018 Nov 4;2018:7902841. doi: 10.1155/2018/7902841. eCollection 2018. Mediators Inflamm. 2018. PMID: 30524198 Free PMC article.
-
LRP1 protects the vasculature by regulating levels of connective tissue growth factor and HtrA1.Arterioscler Thromb Vasc Biol. 2013 Sep;33(9):2137-46. doi: 10.1161/ATVBAHA.113.301893. Epub 2013 Jul 18. Arterioscler Thromb Vasc Biol. 2013. PMID: 23868935 Free PMC article.
-
Smooth muscle LDL receptor-related protein-1 inactivation reduces vascular reactivity and promotes injury-induced neointima formation.Arterioscler Thromb Vasc Biol. 2009 Nov;29(11):1772-8. doi: 10.1161/ATVBAHA.109.194357. Epub 2009 Sep 3. Arterioscler Thromb Vasc Biol. 2009. PMID: 19729608 Free PMC article.
-
Low-density lipoprotein receptor-related protein-1: role in the regulation of vascular integrity.Arterioscler Thromb Vasc Biol. 2014 Mar;34(3):487-98. doi: 10.1161/ATVBAHA.113.301924. Epub 2014 Feb 6. Arterioscler Thromb Vasc Biol. 2014. PMID: 24504736 Free PMC article. Review.
-
The molecular mechanism of LRP1 in physiological vascular homeostasis and signal transduction pathways.Biomed Pharmacother. 2021 Jul;139:111667. doi: 10.1016/j.biopha.2021.111667. Epub 2021 May 7. Biomed Pharmacother. 2021. PMID: 34243608 Review.
Cited by
-
Aortic Cellular Diversity and Quantitative Genome-Wide Association Study Trait Prioritization Through Single-Nuclear RNA Sequencing of the Aneurysmal Human Aorta.Arterioscler Thromb Vasc Biol. 2022 Nov;42(11):1355-1374. doi: 10.1161/ATVBAHA.122.317953. Epub 2022 Sep 29. Arterioscler Thromb Vasc Biol. 2022. PMID: 36172868 Free PMC article.
-
Transcriptome-based identification of new anti-inflammatory and vasodilating properties of the n-3 fatty acid docosahexaenoic acid in vascular endothelial cell under proinflammatory conditions [corrected].PLoS One. 2015 Jun 26;10(6):e0129652. doi: 10.1371/journal.pone.0129652. eCollection 2015. PLoS One. 2015. PMID: 26114549 Free PMC article.
-
Myeloid cell receptor LRP1/CD91 regulates monocyte recruitment and angiogenesis in tumors.Cancer Res. 2013 Jul 1;73(13):3902-12. doi: 10.1158/0008-5472.CAN-12-4233. Epub 2013 Apr 30. Cancer Res. 2013. PMID: 23633492 Free PMC article.
-
Macrophage LRP1 Promotes Diet-Induced Hepatic Inflammation and Metabolic Dysfunction by Modulating Wnt Signaling.Mediators Inflamm. 2018 Nov 4;2018:7902841. doi: 10.1155/2018/7902841. eCollection 2018. Mediators Inflamm. 2018. PMID: 30524198 Free PMC article.
-
Role of the LDL Receptor-Related Protein 1 in Regulating Protease Activity and Signaling Pathways in the Vasculature.Curr Drug Targets. 2018;19(11):1276-1288. doi: 10.2174/1389450119666180511162048. Curr Drug Targets. 2018. PMID: 29749311 Free PMC article. Review.
References
-
- Strickland DK, Ashcom JD, Williams S, Burgess WH, Migliorini M, et al. Sequence identity between the a2-macroglobulin receptor and low density lipoprotein receptor-related protein suggests that this molecule is a multifunctional receptor. J Biol Chem. 1990;265:17401–17404. - PubMed
-
- Moestrup SK, Gliemann J. Purification of the Rat Hepatic a2-Macroglobulin Receptor as an Approximately 440 kDa Single Chain Polypeptide. J Biol Chem. 1989;264:15574–15577. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

