Utilization of murine colonoscopy for orthotopic implantation of colorectal cancer
- PMID: 22174916
- PMCID: PMC3236220
- DOI: 10.1371/journal.pone.0028858
Utilization of murine colonoscopy for orthotopic implantation of colorectal cancer
Abstract
Background: Colorectal-cancer (CRC) research has greatly benefited from the availability of small animal tumor models. Spontaneous and chemically-induced CRC models are widely used yet limited in their resemblance to human disease and are often prolonged, not accurately repetitive, and associated with inflammatory side effects. In-situ murine or human tumor implantation in the gastrointestinal tract of mice is extremely challenging, and limited by inter-animal variability and procedure-related complications and mortality. As a result, in frequent studies CRC is implanted in distal sites, most commonly the subcutaneous region, an approach that is greatly limited by the absence of normal gastrointestinal tumor milieu and has substantial effects on tumor development.
Aims: In this study we aimed to develop a well-tolerated repetitive tool to study CRC in small animals by adapting the murine colonoscopy system to serve as a platform for colonic sub-mucosal orthotopic implantation of human and murine CRC tumor cells.
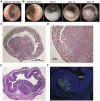
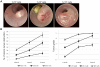
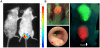
Results: We report the establishment of a novel small-animal CRC model that is minimally invasive, rapid, well-tolerated, highly reproducible, and confers precise control of tumor number, location and growth rate. Moreover, we show that this model uniquely allows the side-by-side induction of distinct genetically manipulated tumors, enabling the mechanistic study of tumor interaction and cross-talk within the native intestinal microenvironment.
Conclusions: Employment of this new approach may represent a major technical advance for the in-vivo study of CRC.
Conflict of interest statement
Figures
Similar articles
-
Mouse models of colorectal cancer: Past, present and future perspectives.World J Gastroenterol. 2020 Apr 7;26(13):1394-1426. doi: 10.3748/wjg.v26.i13.1394. World J Gastroenterol. 2020. PMID: 32308343 Free PMC article. Review.
-
Endoscopy-guided orthotopic implantation of colorectal cancer cells results in metastatic colorectal cancer in mice.Clin Exp Metastasis. 2016 Aug;33(6):551-62. doi: 10.1007/s10585-016-9797-7. Epub 2016 May 4. Clin Exp Metastasis. 2016. PMID: 27146063
-
Patient-derived Orthotopic Xenograft Models for Human Urothelial Cell Carcinoma and Colorectal Cancer Tumor Growth and Spontaneous Metastasis.J Vis Exp. 2019 May 12;(147). doi: 10.3791/59223. J Vis Exp. 2019. PMID: 31132059
-
A surgical orthotopic organoid transplantation approach in mice to visualize and study colorectal cancer progression.Nat Protoc. 2018 Feb;13(2):235-247. doi: 10.1038/nprot.2017.137. Epub 2018 Jan 4. Nat Protoc. 2018. PMID: 29300390
-
Colorectal cancer models for novel drug discovery.Expert Opin Drug Discov. 2015;10(11):1217-29. doi: 10.1517/17460441.2015.1079618. Epub 2015 Aug 21. Expert Opin Drug Discov. 2015. PMID: 26295972 Free PMC article. Review.
Cited by
-
Biology and significance of circulating and disseminated tumour cells in colorectal cancer.Langenbecks Arch Surg. 2012 Apr;397(4):535-42. doi: 10.1007/s00423-012-0917-9. Epub 2012 Feb 15. Langenbecks Arch Surg. 2012. PMID: 22350614 Review.
-
IL-1R signaling drives enteric glia-macrophage interactions in colorectal cancer.Nat Commun. 2024 Jul 19;15(1):6079. doi: 10.1038/s41467-024-50438-2. Nat Commun. 2024. PMID: 39030280 Free PMC article.
-
Cancer cell-associated fatty acid synthase activates endothelial cells and promotes angiogenesis in colorectal cancer.Carcinogenesis. 2014 Jun;35(6):1341-51. doi: 10.1093/carcin/bgu042. Epub 2014 Feb 7. Carcinogenesis. 2014. PMID: 24510238 Free PMC article.
-
Mouse models of colorectal cancer: Past, present and future perspectives.World J Gastroenterol. 2020 Apr 7;26(13):1394-1426. doi: 10.3748/wjg.v26.i13.1394. World J Gastroenterol. 2020. PMID: 32308343 Free PMC article. Review.
-
Development of a colon cancer GEMM-derived orthotopic transplant model for drug discovery and validation.Clin Cancer Res. 2013 Jun 1;19(11):2929-40. doi: 10.1158/1078-0432.CCR-12-2307. Epub 2013 Feb 12. Clin Cancer Res. 2013. PMID: 23403635 Free PMC article.
References
-
- Jemal A, Siegel R, Ward E, Murray T, Xu J, et al. Cancer statistics, 2006. CA: a cancer journal for clinicians. 2006;56:106–130. - PubMed
-
- Taketo MM, Edelmann W. Mouse models of colon cancer. Gastroenterology. 2009;136:780–798. - PubMed
-
- Edelmann L, Edelmann W. Loss of DNA mismatch repair function and cancer predisposition in the mouse: animal models for human hereditary nonpolyposis colorectal cancer. American journal of medical genetics Part C, Seminars in medical genetics. 2004;129C:91–99. - PubMed
-
- Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–324. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

