Rosiglitazone inhibits transforming growth factor-β1 mediated fibrogenesis in ADPKD cyst-lining epithelial cells
- PMID: 22174924
- PMCID: PMC3235173
- DOI: 10.1371/journal.pone.0028915
Rosiglitazone inhibits transforming growth factor-β1 mediated fibrogenesis in ADPKD cyst-lining epithelial cells
Abstract
Background: Interstitial fibrosis plays an important role in progressive renal dysfunction in autosomal dominant polycystic kidney disease (ADPKD). In our previous studies, we confirmed that PPAR-γ agonist, rosiglitazone could protect renal function and prolong the survival of a slowly progressive ADPKD animal model by reducing renal fibrosis. However, the mechanism remains unknown.
Methods: Primary culture epithelial cells pretreated with TGF-β1 were incubated with rosiglitazone. Extracellular matrix proteins were detected using real-time PCR and Western blotting. MAPK and Smad2 phosphorylation were measured with western blot. ERK1/2 pathway and P38 pathway were inhibited with the specific inhibitors PD98059 and SB203580. The Smad2 pathway was blocked with the siRNA. To address whether PPAR-γ agonist-mediated inhibition of TGF-β1-induced collagen type I expression was mediated through a PPAR-γ dependent mechanism, genetic and pharmaceutical approaches were used to block the activity of endogenous PPARγ.
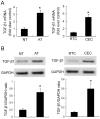
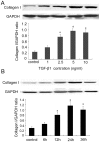
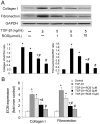
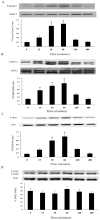
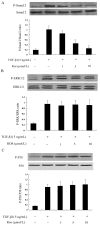
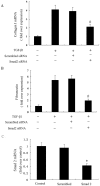
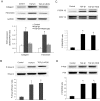
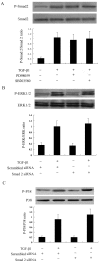
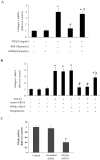
Results: TGF-β1-stimulated collagen type I and fibronectin expression of ADPKD cyst-lining epithelia were inhibited by rosiglitazone in a dosage-dependent manner. Smad2, ERK1/2 and P38 pathways were activated in response to TGF-β1; however, TGF-β1 had little effect on JNK pathway. Rosiglitazone suppressed TGF-β1 induced Smad2 activation, while ERK1/2 and P38MAPK signals remained unaffected. Rosiglitazone could also attenuate TGF-β1-stimulated collagen type I and fibronectin expression in primary renal tubular epithelial cells, but had no effect on TGF-β1-induced activation of Smad2, ERK1/2 and P38 pathways. There was no crosstalk between the Smad2 and MAPK pathways in ADPKD cyst-lining epithelial cells. These inhibitory effects of rosiglitazone were reversed by the PPARγ specific antagonist GW9662 and PPARγ siRNA.
Conclusion: ADPKD cyst-lining epithelial cells participate in TGF-β1 mediated fibrogenesis. Rosiglitazone could suppress TGF-β1-induced collagen type I and fibronectin expression in ADPKD cyst-lining epithelia through modulation of the Smad2 pathway. Our study may provide therapeutic basis for clinical applications of rosiglitazone in retarding the progression of ADPKD.
Conflict of interest statement
Figures

References
-
- Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369:1287–1301. - PubMed
-
- Torres VE, Harris PC. Mechanisms of Disease: autosomal dominant and recessive polycystic kidney diseases. Nat Clin Pract Nephrol 2: 40-55; quiz. 2006;55 - PubMed
-
- Lieske JC, Toback FG. Autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1993;3:1442–1450. - PubMed
-
- Hassane S, Leonhard WN, van der Wal A, Hawinkels LJ, Lantinga-van Leeuwen IS, et al. Elevated TGFbeta-Smad signalling in experimental Pkd1 models and human patients with polycystic kidney disease. J Pathol. 2010;222:21–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

