TTF-1 action on the transcriptional regulation of cyclooxygenase-2 gene in the rat brain
- PMID: 22174936
- PMCID: PMC3236776
- DOI: 10.1371/journal.pone.0028959
TTF-1 action on the transcriptional regulation of cyclooxygenase-2 gene in the rat brain
Abstract
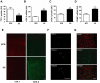
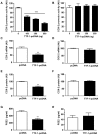
We have recently found that thyroid transcription factor-1 (TTF-1), a homeodomain-containing transcription factor, is postnatally expressed in discrete areas of the hypothalamus and closely involved in neuroendocrine functions. We now report that transcription of cyclooxygenase-2 (COX-2), the rate limiting enzyme in prostaglandin biosynthesis, was inhibited by TTF-1. Double immunohistochemistry demonstrated that TTF-1 was expressed in the astrocytes and endothelial cells of blood vessel in the hypothalamus. Promoter assays and electrophoretic mobility shift assays showed that TTF-1 inhibited COX-2 transcription by binding to specific binding domains in the COX-2 promoter. Furthermore, blocking TTF-1 synthesis by intracerebroventricular injection of an antisense oligomer induced an increase of COX-2 synthesis in non-neuronal cells of the rat hypothalamus, and resulted in animals' hyperthermia. These results suggest that TTF-1 is physiologically involved in the control of thermogenesis by regulating COX-2 transcription in the brain.
Conflict of interest statement
Figures




Similar articles
-
Regulation of pituitary adenylate cyclase-activating polypeptide gene transcription by TTF-1, a homeodomain-containing transcription factor.J Biol Chem. 2002 Sep 27;277(39):36863-71. doi: 10.1074/jbc.M206443200. Epub 2002 Jul 16. J Biol Chem. 2002. PMID: 12122016
-
TTF-1, a homeodomain-containing transcription factor, participates in the control of body fluid homeostasis by regulating angiotensinogen gene transcription in the rat subfornical organ.J Biol Chem. 2003 Jul 18;278(29):27043-52. doi: 10.1074/jbc.M303157200. Epub 2003 May 2. J Biol Chem. 2003. PMID: 12730191
-
Thyroid transcription factor-1 regulates feeding behavior via melanocortin pathway in the hypothalamus.Diabetes. 2011 Mar;60(3):710-9. doi: 10.2337/db10-0183. Epub 2011 Jan 31. Diabetes. 2011. PMID: 21282365 Free PMC article.
-
Thyroid transcription factor-1 facilitates cerebrospinal fluid formation by regulating aquaporin-1 synthesis in the brain.J Biol Chem. 2007 May 18;282(20):14923-31. doi: 10.1074/jbc.M701411200. Epub 2007 Mar 19. J Biol Chem. 2007. PMID: 17371871
-
Thyroid transcription factor-1 (TTF-1/Nkx2.1/TITF1) gene regulation in the lung.Clin Sci (Lond). 2009 Jan;116(1):27-35. doi: 10.1042/CS20080068. Clin Sci (Lond). 2009. PMID: 19037882 Review.
Cited by
-
Systematic study of constitutive cyclooxygenase-2 expression: Role of NF-κB and NFAT transcriptional pathways.Proc Natl Acad Sci U S A. 2016 Jan 12;113(2):434-9. doi: 10.1073/pnas.1517642113. Epub 2015 Dec 28. Proc Natl Acad Sci U S A. 2016. PMID: 26712011 Free PMC article.
-
Sirtuin1-Mediated Deacetylation of Hypothalamic TTF-1 Contributes to the Energy Deficiency Response.Int J Mol Sci. 2023 Aug 7;24(15):12530. doi: 10.3390/ijms241512530. Int J Mol Sci. 2023. PMID: 37569904 Free PMC article.
-
Insulin sensing by astrocytes is critical for normal thermogenesis and body temperature regulation.J Endocrinol. 2020 Oct;247(1):39-52. doi: 10.1530/JOE-20-0052. J Endocrinol. 2020. PMID: 32698146 Free PMC article.
-
A Role for the Transcription Factor Nk2 Homeobox 1 in Schizophrenia: Convergent Evidence from Animal and Human Studies.Front Behav Neurosci. 2016 Mar 30;10:59. doi: 10.3389/fnbeh.2016.00059. eCollection 2016. Front Behav Neurosci. 2016. PMID: 27064909 Free PMC article.
-
α-Spinasterol: a COX inhibitor and a transient receptor potential vanilloid 1 antagonist presents an antinociceptive effect in clinically relevant models of pain in mice.Br J Pharmacol. 2017 Dec;174(23):4247-4262. doi: 10.1111/bph.13992. Epub 2017 Oct 18. Br J Pharmacol. 2017. PMID: 28849589 Free PMC article.
References
-
- Boulant JA. Role of the preoptic-anterior hypothalamus in thermoregulation and fever. Clin Infect Dis. 2000;31(Suppl 5):S157–161. - PubMed
-
- Conti B, Tabarean I, Andrei C, Bartfai T. Cytokines and fever. Front Biosci. 2004;9:1433–1449. - PubMed
-
- Li S, Wang Y, Matsumura K, Ballou LR, Morham SG, et al. The febrile response to lipopolysaccharide is blocked in cyclooxygenase-2 (-/-), but not in cyclooxygenase-1 (-/-) mice. Brain Res. 1999;825:86–94. - PubMed
-
- Ushikubi F, Segi E, Sugimoto Y, Murata T, Matsuoka T, et al. Impaired febrile response in mice lacking the prostaglandin E receptor subtype EP3. Nature. 1998;395:281–284. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

